��2012?������һģ����ͼΪʵ�����г����������Ʊ����������ռ�������ʵ��IJ����������Ը�����ĿҪ�ش��������⣺
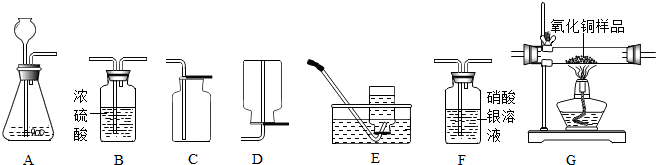
��1������ʯ��ʯ��ϡ����Ϊԭ����ȡ������̼���壬�������ӷ����������Ȼ������壬������ȡ�����岻������Ҫ�ռ�һƿ��������Ķ�����̼���壮
����ѡ����������˳��Ϊ
AFBC
AFBC
����д���������ĸ����
�����ɶ�����̼ʱ����������Ӧ�Ļ�ѧ����ʽΪ��
CaCO3+2HCl=CaCl2+H2O+CO2��
CaCO3+2HCl=CaCl2+H2O+CO2��
��
���������F���
��ɫ����
��ɫ����
������֤��������̼�л����Ȼ������壮
��ʵ������ȡ������̼���ʣ���Һ�У�������ʣ����������ᣬ����֤����Ĵ��ڣ����AgNO
3��Һ��ʯ�Na
2CO
3��Һ�����Լ���ѡ��һ���Լ�������֤��������ѡ�������
ѡNa2CO3��Һ����Ϊ������̼���Ʒ�Ӧ������ð������������
ѡNa2CO3��Һ����Ϊ������̼���Ʒ�Ӧ������ð������������
��
��2��������A��ʢ��Zn��H
2SO
4��Һ��ijͬѧ�������Ʊ����������ⶨCuO��Ʒ��CuO�Ĵ��ȣ����ʲ���Ӧ��������������˳��ΪA��G��B������֪��CuO+H
2Cu+H
2O��
��ʵ��ʱ����۲쵽װ��G�еĺ�ɫ������
��
��
ɫ��
�ڸ�ͬѧͨ��������Ӧǰ��Bװ�����������ӣ���������Ʒ��CuO�Ĵ��ȣ����������
ƫ��
ƫ��
���ƫ����ƫС����������Ӱ�족֮һ����



 ��ɢ˼ά�¿���ϵ�д�
��ɢ˼ά�¿���ϵ�д�
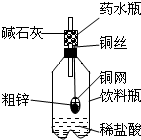 ijͬѧ�����ճ������г�������Ʒ������һ����ͼװ�ã��ø�װ�öԴ�п��Ʒ����ʵ�飮������������ʵ�鱨��
ijͬѧ�����ճ������г�������Ʒ������һ����ͼװ�ã��ø�װ�öԴ�п��Ʒ����ʵ�飮������������ʵ�鱨��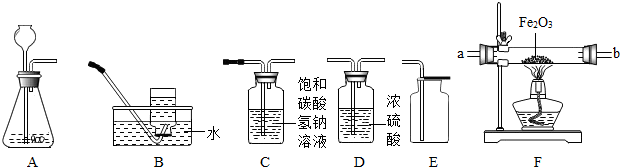

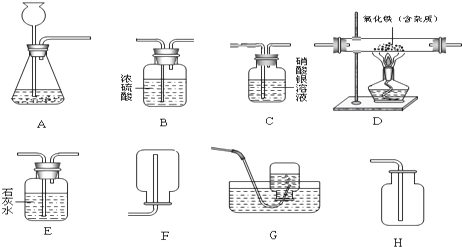
 ijͬѧ�����ճ������г�������Ʒ������һ����ͼװ�ã��ø�װ�öԴ�п��Ʒ����ʵ�飮������������ʵ�鱨�森
ijͬѧ�����ճ������г�������Ʒ������һ����ͼװ�ã��ø�װ�öԴ�п��Ʒ����ʵ�飮������������ʵ�鱨�森