
�����õ������ԭ��������Ca:40 Cl:35.5 H:1 O:16 C:12
48�����Ļ���ˮ�೧������ˮ�೩�����أ�������ԭ��֮һ��ʯ��ʯ��ij��ѧ��ȤС���ʯ��ʯ����̽����ȡ��ʯ��ʯ��Ʒ16g����200gϡ������Ĵμ��룬���������������ݼ��±�(��֪ʯ��ʯ��Ʒ�к��е����ʲ�����ˮҲ����ϡ���ᷴӦ)������㣺
| ��� | ����ϡ�����������g | �������������g |
| ��1�� | 50 | 11 |
| ��2�� | 50 | 6 |
| ��3�� | 50 | 4.8 |
| ��4�� | 50 | m |
 �����Ƹ���ʦ����ϵ�д�
�����Ƹ���ʦ����ϵ�д� ��ͨ����ͬ����ϰ��ϵ�д�
��ͨ����ͬ����ϰ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��1������ǰ��ƿŨ������Һ�������Ƕ��٣�
��1������ǰ��ƿŨ������Һ�������Ƕ��٣��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
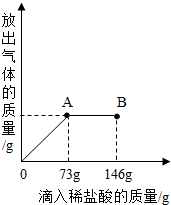 ��֪ Na2CO3��ˮ��Һ�ʼ��ԣ���һ�ձ���ʢ��20.4g Na2CO3��NaCl ��ɵĹ�������������μ������ʷ���Ϊ10%��ϡ���ᣮ�ų��������������������ϡ�����������ϵ������ͼ��ʾ�����������ش����⣺
��֪ Na2CO3��ˮ��Һ�ʼ��ԣ���һ�ձ���ʢ��20.4g Na2CO3��NaCl ��ɵĹ�������������μ������ʷ���Ϊ10%��ϡ���ᣮ�ų��������������������ϡ�����������ϵ������ͼ��ʾ�����������ش����⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011�����ʡ�人�г����һѧУ�п���ѧģ���Ծ���ʮ�ģ��������棩 ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�����ʡ�人�к������п���ѧģ���Ծ��������������棩 ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2008�����ʡ�������п���ѧ�Ծ����α�����������棩 ���ͣ������
 ��2008?��������1������ǰ��ƿŨ������Һ�������Ƕ��٣�
��2008?��������1������ǰ��ƿŨ������Һ�������Ƕ��٣��鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com