
����Ŀ��ˮ����Һ��������������������Ҫ�����á�
(1)�������ʷ���������ˮ���������ӵ���ʽ��ɢ�γɾ�һ��Һ����____(�����)��
A ���� B ���� C ʳ�� D ̼���
(2) �ס��ҡ������ֹ���������ˮ�е��ܽ��������ͼ��ʾ��
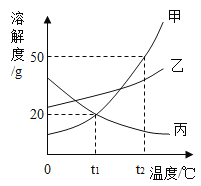
�ٱ���Һ�����ʿ�����______(�����)��
A NaCl B Ca(OH)2 C KNO3
��t1��ʱ�����ܽ����______��
��t2��ʱ����50gˮ�м���30g���壬��ֽ��裬���γ�_____g����Һ��
�������й�������ȷ����________(�����)��
A t1��ʱ����50�˼ͱ��������Ƴɵı�����Һ����Ҫˮ�������ף���
B �ֱ������ļ��ҵı�����Һ��t2�潵��t1��ʱ��������Һ�������ң���
C ���ӽ����͵ı���Һת��ɱ�����Һʱ����Һ��������������һ������
D �����л���������ʱ���ɲ��ý��½ᾧ�ķ����ᴿ��
���𰸡�C B 20g 75 BD
��������
��1��A����������ˮ���γ��Է�����ʽ���ڵ���Һ�����������⣻
B�����Ͳ�����ˮ�������γ���Һ�����������⣻
C��ʳ������ˮ���γ��������Ӻ���������ʽ���ڵ���Һ���������⣻
D��̼��Ʋ�����ˮ�����������⡣��ѡC��
��2������ͼ�п��Կ�������Һ���ܽ�����¶ȵ����߶���С���ʿ������������ƣ��Ȼ��Ƶ��ܽ�����¶ȱ仯��������ص��ܽ�����¶ȵ����߶����ߣ���ѡB��
����ͼ�п��Կ���t1��ʱ�����ܽ����20g��
����ͼ�п��Կ�����t2��ʱ�����ܽ����50g����50gˮֻ���ܽ�25g�ף��ʳ�ֽ������Һ������Ϊ50g+25g=75g��
��A��t1��ʱ�ͱ����ܽ��һ�������üͱ��������óɵı�����Һ��Ҫˮ��������ͬ������
B����ͼ�п��Կ����ֱ������ļ��ҵı�����Һ��t2������t1��ʱ���������Ĺ����������࣬��������Һ�������ң��ף���ȷ��
C�����ӽ����͵ı���Һת��ɱ�����Һʱ�����ֻ�����¶����Ǽ����ʵĻ�����Һ����������������һ��������
D�����ܽ�����¶ȱ仯���ȱ��ҵĸ��ʵ����л���������ʱ���ɲ��ý��½ᾧ�ķ����ᴿ�ף���ȷ��
��ѡBD��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Һ����������������������ء���ش��������⣺
��1������ˮƿ����ˮ�Զ��������˵��������ˮ�е��ܽ����_____�йء�
��2���ס��ҡ������ֹ������ʣ������ᾧˮ���Ҳ���ˮ��Ӧ�����ܽ����������ͼ��ʾ��
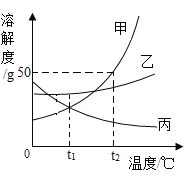
��t2��ʱ���ס��ҡ����������ʵ��ܽ���ɴ�С��˳����_____��
��t2��ʱ����50g����50gˮ���ܵõ��ı�����Һ��������������Һ��������Ϊ_____��
�۽����ı�����Һ��Ϊ��������Һ����������Һ�������䣬���Բ���_____������
��t2��ʱ���ס��ҡ����������ʵı�����Һ������t1��ʱ����������Һ���������������ɴ�С��ϵ��_____��
��3����һ������������������Ϊ20%���������Һ��ͬʱ����4g����غ�16gˮ���õ���Һ��������������Ϊ_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���á�������������������գ�
��1���ؿ���Ԫ�غ�������Ԫ��_____��Ԫ�أ�
��2��C2H6O��̼Ԫ�ص���������_____CH2O��̼Ԫ�ص�����������
��3��50������ˮ��50�����ľƾ���Ϻ�����_____100������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���±�ΪԪ�����ڱ��в���Ԫ�ص������Ϣ���������±��ش�������⣺
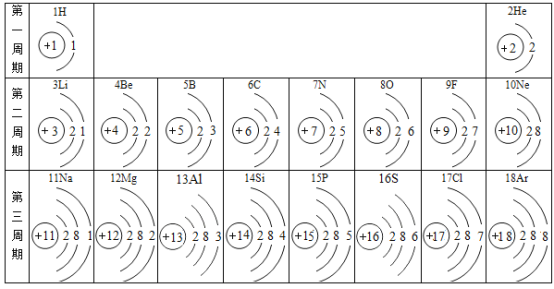
��1����15��Ԫ�ص����� __________��
��2��17��Ԫ�ص�ԭ���ڻ�ѧ��Ӧ�бȽ�����__________��ѡ������������ʧ�������ӱ�������ӡ������ӷ�����__________
��3����16��Ԫ�غ�8��Ԫ���γɻ�����Ļ�ѧʽ��__________��
��4������ͼ�����ܻ�ȡ����Ϣ��___________��дһ��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪�����¡�C���ġ��ų��г����ĵ��ʻ��������ͬһ��Ԫ�أ�����B��һ���ж������壬����һ�ְ�ɫ�����E��ʹ��ɫʯ����ɫ������ʯ��ˮ����Ҫ�ɷ֣���������ͼ��ת����ϵ��
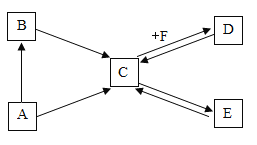
��1����д���������ʵĻ�ѧʽ��A______��B_______ ��D_______
��2��д������ת���Ļ�ѧ��Ӧ����ʽ��
C![]() E��_______________��������Ӧ���ͣ�___________��
E��_______________��������Ӧ���ͣ�___________��
C![]() D��_____________
D��_____________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ҷ��ӷ�Ӧ���ɱ����ӵ�ʾ��ͼ����,����˵������ȷ����( )
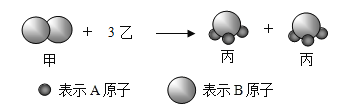
A.���ʼ��ǵ���
B.��������A��Bԭ�Ӹ�����Ϊ3:1
C.��Ӧǰ��ԭ�ӵĸ���û�иı䣬���ӵĸ���Ҳû�иı�
D.���������غ㶨�ɿ���֪�ҵĻ�ѧʽΪA2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������Ľṹʾ��ͼ����ʾ��ͼ���:
��1����ͼ1,A��B���������Ľṹʾ��ͼ,��ش���������:

����A��ijԪ�ص�ԭ��,��x=_____��
����B��ij�����ӵĽṹʾ��ͼ,��Y����ֵ�����������е�_____��
A 12
B 11
C 10
D 9
��2����һ�������£�A��B�ܷ�����ѧ��Ӧ����C��D,����ʾ��ͼ��ͼ2:
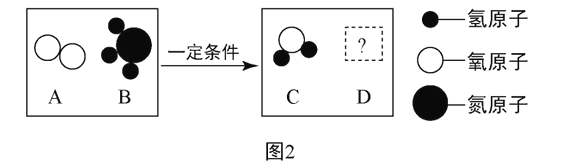
����DΪ���������������������,��ͼ2������������,���ڵ��ʵ���_____���ѧʽ����.
����DΪ��Ԫ�ص�+2��������,��A��B�ķ��Ӹ�����Ϊ_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��B��C��D�dz��л�ѧ���������ʣ������������о�����ͬһ��Ԫ�ء�����AΪ��ɫ���嵥�ʣ�B��CΪ�����D�Ǵ���ʯ����Ҫ�ɷ֡�����֮��IJ���ת����ϵ��ͼ��ʾ��ͼ�з�Ӧ���������ַ�Ӧ���������ʡ�ԣ���
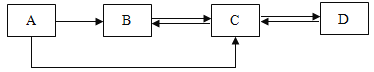
д������A�Ļ�ѧʽ��A_____������D����;��_____��д��������Bת��Ϊ����C�Ļ�ѧ����ʽ��_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������ũҵ�������벻��ˮ����ͼ������ˮ������ˮ��ʾ��ͼ��
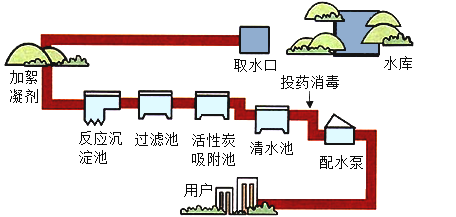
��1������ˮ����������ˮʱ��ʹ�õľ�ˮ������ ___________������ţ���
A ���� B ���� C ��� D ���� E ����
��2��������Ϊ���ڽ�Լ��ˮ����_________������ţ���
A �����������ֹر�ˮ��ͷ B ϴ��˵�ˮ��������
C ����ϵط�ˮˢ�� D ũҵ��Ϊ�˹�ȸ���֣�ͨ�����ô�ˮ����
��3���������ƣ�Na2FeO4����һ�����͵ľ�ˮ����������Ԫ�صĻ��ϼ�Ϊ_________��
��4���ù�ҵ�����Ƴɵ�ҽ�������������л��е��ؽ������������к�������ĸ���ָ_________ ������Ԫ������������������ԭ��������
��5����������_________����Ӳˮ����ˮ��
��6��X��һ�����͵�����ˮ����������ҵ����ȡX�Ļ�ѧ����ʽΪ��Cl2+2NaClO2=2 X +2NaCl����X�Ļ�ѧʽΪ_________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com