

���� ��1���ݵ��ˮʵ���и����������������������������
��2������ȼ��ʵ���ע������Ͷ�������������ˮ���
��3��������Һ���γɹ��̺���Һ��������������
��4�������ܽ�������������ֵ��������������
��5�������ʵ�����=��Һ�����������ʵ���������������������Ƶ����������� ����ʽ����������������
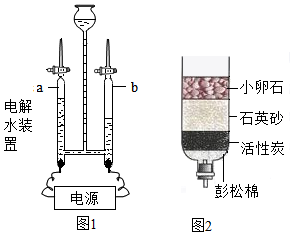
��� �⣺��1�����ˮʵ���и��������������������������������������������2����ͼʾ��a�Թ����ӵ��������������٣���������
��2����ȼ��ʵ����ƿ�з�ˮ��Ϊ���������ɵĶ�������ֹ��Ⱦ������������������ˮ�γ��ᣬ�����ԣ�pH��7��
��3����ۡ�ֲ���Ͳ�������ˮ���������ʾ�������ˮ���γɾ�һ���ȶ��Ļ����--��Һ���ʲ����γ���Һ����AD��
��4����t1��ʱ�������ʵ��ܽ��Ϊ 20g��
��t2��C ʱ���ܽ����50g����100gˮ������ܽ�50g�ļף����Խ�30g�����ʼ��뵽50gˮ�У�����ܽ⣬����ܽ�25g��������Һ������Ϊ75g��
�۽�t2��Cʱ�ס��ҡ����������ʵı�����Һ���µ�t1��C�����ҵ��ܽ�ȼ�С�������壬�����ܽ������Ϊ��������Һ��������Һ�����������ļ���ʽ$\frac{�ܽ��}{�ܽ��+100g}$��100%�����ܽ�ȴ������ʵ��������������º��ҵ��ܽ�ȴ��ڼ��ܽ�ȴ��ڽ���ǰ�����ܽ�ȣ���������Һ�����ʵ�����������С��ϵΪ�ң��ף�����
��5�����ĵ�����������Һ�����ʵ�����Ϊ��40g��15%=6g
��һ����ʯ�Ͳ�Ʒ�ﺬH2SO4������Ϊx
2NaOH+H2SO4�TNa2SO4+2H2O
80 98
6g x
$\frac{80}{98}$=$\frac{6g}{x}$
x=7.35g
������һ������ʯ�Ͳ�Ʒ�к�����7.35��
�ʴ�Ϊ����1����������2���������ɵĶ�������ֹ��Ⱦ������С�ڣ���3��AD����4����20g����75g�����ң��ף�����
��5�����ĵ�����������Һ�����ʵ�����Ϊ��40g��15%=6g
��һ����ʯ�Ͳ�Ʒ�ﺬH2SO4������Ϊx
2NaOH+H2SO4�TNa2SO4+2H2O
80 98
6g x
$\frac{80}{98}$=$\frac{6g}{x}$
x=7.35g
������һ������ʯ�Ͳ�Ʒ�к�����7.35�ˣ�
���� �����ѶȲ�����Ҫ�����˹����ܽ����������ʾ�����壬��ȼ��ʵ�顢����ʽ����ؼ��㡢��Һ���γɡ����ˮ�����֪ʶ�ȣ��ܽϺ�����ѧ��Ӧ��֪ʶ��������������


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ϳ��� | B�� | �ϳ���ά | C�� | ��Ȼ��ά | D�� | ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ֲ����� | B�� | ϴ�Ӽ��黯���� | C�� | ����̿��ˮ | D�� | �ɱ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��Դ�뻷����Ϊ���������ע�����⣮
��Դ�뻷����Ϊ���������ע�����⣮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
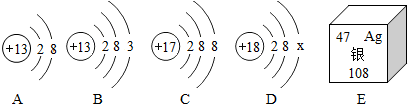
| A�� | 14 | B�� | 0 | C�� | 7 | D�� | 10 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ��̽����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | Na 2CO 3��Fe��OH�� 3��Zn��Fe 2O 3 | B�� | SO 3��Cu��BaCl 2��HCl | ||
| C�� | Zn��Fe��OH�� 3��KNO 3��CuO | D�� | SO 3��Cu��NaCl��CuO |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com