
����Ŀ����84����Һ����������Һ��Ӧ������ȡ��������Ӧ����ʽΪNaClO+NaCl+H2SO4![]() Na2SO4+Cl2��+
Na2SO4+Cl2��+
H2O��Ϊ̽�����������ʣ�ijͬѧ�����������ʾ��ʵ��װ�á�
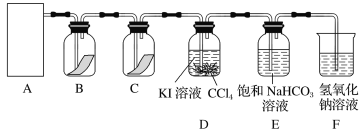
��ش�
(1)�ڸ�ʵ���У���ȡ������װ����________(����ĸ)��
(2)װ��B��C�����ηŵ��Ǹ���ĺ�ɫ������ʪ��ĺ�ɫ������ʵ������и�ͬѧ����װ��B�еIJ���Ҳ��ɫ����ԭ�������________________________________________________������������ĸĽ�����_______________________________________________��
(3)D�е�������_____________________����Ӧ�����ӷ���ʽΪ_________________________________��
����D��Һ��ķ�����___________________________________________��
(4)д��������NaOH��Һ��Ӧ�����ӷ���ʽ______________________________���÷�Ӧ���������뻹ԭ�������ʵ���֮��Ϊ________��Ϊ��֤β�����պ����Һ�д���Cl����ȷ�IJ�����____________________��
(5)����ͨ�뱥��NaHCO3��Һ�ܲ�����ɫ���壬��֪���ԣ����̼������ᣬ��ʵ��֤��Cl2��H2O��Ӧ�IJ����к���________��
���𰸡�(1)c
(2)Cl2�л�������H2O(g) ��A��B֮������װ��ŨH2SO4��ϴ��ƿ
(3)�²�CCl4����Ϻ�ɫ Cl2+2I===I2+2Cl ��Һ
(4)Cl2+2OH===Cl+ClO+H2O 1��1 ȡ�����ձ�������Cl2�����Һ������������ϡHNO3�ữ���ټ���AgNO3��Һ�����а�ɫ�������ɣ�֤�����к���Cl
(5)����
��������(1)���ݡ���+Һ![]() ����ԭ����֪Ӧѡ��cװ����ȡCl2��
����ԭ����֪Ӧѡ��cװ����ȡCl2��
(2)������ɫ֤��Cl2�л���H2O(g)��������HClO��Ӧ���ӳ�H2O(g)��װ�á�
(3)Cl2+2I===I2+2Cl��CCl4��I2��ȡ������I2����CCl4����Һ���Ϻ�ɫ���������ֻ������ܵ�Һ����÷�Һ����
(4)Cl2��NaOH��Һ��Ӧ�����ӷ���ʽΪCl2+2OH===Cl+ClO+H2O�����ݷ�Ӧǰ����Ԫ�ػ��ϼ۱仯��֪Cl2������������������ԭ���������ʵ���֮��Ϊ1��1������Cl��Ӧ����ϡHNO3�ữ�к���Һ�е�OH������ClOת��ΪHClO���ټ�AgNO3��Һ������AgCl��ɫ�������ɣ�֤������Cl��
(5)������HCl��H2CO3��HClO��HCl��NaHCO3��Ӧ��HCl+NaHCO3===NaCl+H2O+CO2������HClO����NaHCO3��Ӧ����������H2O��Ӧ�IJ����к����ᡣ


 ʱ�����������ҵԭ���ܳ�����ϵ�д�
ʱ�����������ҵԭ���ܳ�����ϵ�д� ����νӽ̲���ĩ���Ԥϰ�人������ϵ�д�
����νӽ̲���ĩ���Ԥϰ�人������ϵ�д� ������ҵ��ٳɳ����½������������ϵ�д�
������ҵ��ٳɳ����½������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ������Ϊ10 g���ӵ�����300 m/s��ˮƽ���ٶ�����һ����ֱ�̶���ľ�壬��ľ����ӵ�������ٶ���200 m/s����ľ����Ϊ10 cm�����ӵ���ľ���ƽ����������
С��ͬѧ���������£���ͬ�����Ľⷨ��
���˶�ѧ��ʽ��v2��![]() ��2ax�ã�
��2ax�ã�
a��![]() ��
��![]() m/s2����2.5��103 m/s2
m/s2����2.5��103 m/s2
��ţ�ٵڶ����ɣ�
F��ma��10������2.5��103��N����2.5��104 N��
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ����6�֣�ͼ��װ�ÿ�����̽�������˶������ϸ��ƽ�������棬�ڹ�Ƭ�Ŀ���Ϊd���������֮��ľ���Ϊs�������ӹ����A������ɾ�ֹ�ͷţ��ֱ����ڹ�Ƭͨ�������A��B���õ�ʱ��ΪtA��tB�����ڹ�Ƭͨ������ŵ�ƽ���ٶȱ�ʾ�ڹ�Ƭ��ֱ����ͨ������ŵ�˲ʱ�ٶȡ�
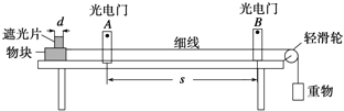
��1������ʵ���в�������������ţ�д������˶��ļ��ٶ�a= ��
��2���ڹ�Ƭͨ������ŵ�ƽ���ٶ� ������ڡ��������ڡ���С�ڡ����ڹ�Ƭ��ֱ����ͨ������ŵ�˲ʱ�ٶȣ��ɴ˻��������д��һ�ּ�С��һ���ķ������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ���������ӷ���ʽ�ͼ�Ҫ���ֱ���������ʵ��
(1)ʢ������Һ���Լ�ƿ�в�������ʱ����˲��״�________________��
(2)ʢNH4F�Լ�Ҫ������ƿ�����ò���ƿ____________________��
(3)�̬���ʲ������ľ�һ���_____________________________��
(4)ʵ�������Ƶ�CuSO4��Һ���������ֻ��ǵ�ԭ����________________(д�����ӷ���ʽ)���ɲ�ȡ������ʱ��������________��ֹ���ǡ�
(5)ijͬѧ���AlCl3(aq)��Na2S(aq)�����ȡAl2S3�������õ�һ�ְ�ɫ��״���������һ��Һ���г�������ζ������ų����Խ�����ɴ˽����ԭ��д����Ӧ�����ӷ���ʽ����ָ����ȡ��������ȷ����________________��
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ����6�֣�ijʵ��С������ͼ����ʾ��ʵ��װ�òⶨ�������ٶȣ�С����������������У���ʱװ�ò��С�����Ⱥ�ͨ�������A��B��ʱ��ֱ�ΪtA��tB����С����ͨ�������A��B��ƽ���ٶȱ�ʾ��������ͨ������ŵ�˲ʱ�ٶȣ����������ż�ľ���Ϊh��

��1�������α꿨�߲��������ֱ����������ͼ����ʾ������ֱ��Ϊd=________cm��
��2��С����ͨ�������B��˲ʱ�ٶ�vB=_______���ⶨ�������ٶȵı���ʽΪg=___________������ʵ���в�õ����������ű�ʾ��
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ�����л������У��е��ж�������š�
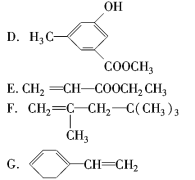
�����������
(1)����������____________(����ţ���ͬ)��
(2)����±��������____________��
(3)���Կ���ȩ����____________��
(4)���Կ���������____________��
(5)�����ڴ��������������____________��
(6)�����ڷ�������������____________��
(7)���ڷ����廯�������____________��
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ�����Ԫ�����ڱ��ش��������⣺
��1�����е�ʵ����Ԫ�����ڱ��IJ��ֱ߽磬����ͼ����ʵ�߲�ȫԪ�����ڱ��ı߽硣

��2����������Ԫ�أ����ڶ�����Ԫ�ص���_______����������Ԫ�ص���_______��gԪ��λ�ڵ�_______����________�壻iԪ��λ�ڵ�_______����__________�塣
��3��Ԫ��f�ǵ�_________���ڡ���_________��Ԫ�أ������ұ߷����а���Ԫ�ص�ʽ��д����Ԫ�ص�ԭ��������Ԫ�ط��š�Ԫ�����ơ����ԭ��������

��4��Ԫ����Ԫ�����ڱ��е�λ����Ԫ��ԭ�ӽṹ�Ĺ�ϵ__________________ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com