
��16�֣�ij��ѧ��ȤС��������з����ⶨNa2SO3��Ʒ��Na2SO4������������
������һ�� �� SO2������������ͼ��ʾװ�ã�ͨ��ʵ��ⶨ����SO2��������
�� SO2������������ͼ��ʾװ�ã�ͨ��ʵ��ⶨ����SO2��������
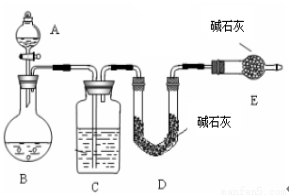
��1��C��ʢ�ŵ��Լ��� ��Eװ�õ������� ��
��2����ʵ��ǰ��ȡm1g��Ʒ���ٲ��SO2������Ϊm2g����ɵ�Na2SO4��������������m2��ͨ��
�ⶨ (��װ�÷���)װ����ʵ��ǰ���������õ��ġ��÷�����ȱ���ǣ�ֻ��һ�㣩
��
���������������������
��һ������ȡm3g��Ʒ������С�ձ��� �ڶ�������С�ձ��м�������ϡ���ᣬ����
������������С�ձ��м�������BaCl2��Һ��Ȼ����ˡ�ϴ�� ���IJ����������������Ϊm4g
��3��������BaCl2�ܷ�ij�Ba(NO3)2�� ����ܡ����ܡ����� �Լ���ԭ��
��
��4������ϴ�ӳ����ķ����� ��
��5����m3=2m4 ��������Na2SO4����������Ϊ ��
��16�֣�ÿ��2�֣�
����һ:
��1��Ũ�����ֹD�м�ʯ�����տ����еĶ�����̼��ˮ����
��2��D SO2��һ����ȫ�����գ�װ�����в���SO2����
������:
��3�����ܣ�����������Ba(NO3)2��SO2������BaSO4
��4����©���ڼ�����ˮ���պý�û��������ˮ��Ȼ�������ټ�ˮϴ��2~3�Ρ�
��5��30.5%
��������
�������������һ����1���������ɵĶ���������������������Ƶ���������������װ��c��ʢ��Ũ��������������װ��E�������Ƿ�ֹD�м�ʯ�����տ����еĶ�����̼��ˮ�������Ӷ�ʹ�����������������ʵ�������
��2��Dװ�õ�����������ֵ��Ϊ�������������������m2��ͨ��װ��D�õ��ģ����÷����IJ���֮���������Ķ�����������ڵ������У�δ����ȫ���գ�
���������÷����Dzⶨ���ᱵ�����������������Ƶ�������������3�����ܸ�Ϊ���ᱵ����Ϊ��Ϊ���ᱵ��Һ���������������¶�������ᱻ�������������ɵij���ȫ�������ᱵ,ʹ���ƫ�ߣ�
��4��ϴ�ӳ����ķ�������©���ڼ�����ˮ���պý�û��������ˮ��Ȼ�������ټ�ˮϴ��2~3�Σ�
��5��m4Ϊ���ᱵ���������������Ƶ�����Ϊm4/233��142g����m3=2m4������Ʒ�������Ƶ���������Ϊm4/233��142/m3��100%=71/233=30.5%��
���㣺�����ʵ��ķ������ۣ����������ļ��㣬������ϴ�Ӳ���


 ��Ӣ���㿨ϵ�д�
��Ӣ���㿨ϵ�д� Ӧ����㲦ϵ�д�
Ӧ����㲦ϵ�д� ״Ԫ����ϵ�д�
״Ԫ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2015�츣��ʡ���ݰ��и߶���ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
���ݱ��渲�ǵ������������������Ӳ�IJ��֣����ű������ݵ����ã�����Ҫ�ɷ�Ϊ�ǻ������[Ca5(PO4)3OH]�������ݱ������������ƽ�⣺ Ca5(PO4)3OH(s)  5Ca2+(aq)��3PO43-(aq)��OH- (aq) Ksp = 6��8��10-37
5Ca2+(aq)��3PO43-(aq)��OH- (aq) Ksp = 6��8��10-37
����˵��������ǣ� ��
A�������������ϵ��Ƿ��ͻ����H+���������������ȣ��
B��������ƽ���֪��С������ʱҪ�ٳ��Ƕಹ��
C������СOH-��Ũ�ȣ�����ƽ�⽫�����ƶ���Ksp��ֵ��Ӧ����
D��ʹ�ú��������ܷ�ֹȣ�ݣ�����ΪCa5(PO4)3OH(s)ת��Ϊ�����ܵ�Ca5(PO4)3F(s)
[ Ca5(PO4)3F(s)��Ksp = 2��8��10-61]
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�����ʡ������ѧ�ڵ�һ�μ�⿼�Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
������������һ���������ܹ��棬��������Ӧ�Լ���ᷢ����ѧ�仯�����������ӷ���ʽ��ȷ����
ѡ�� | ������ | �����Լ� | �����Լ�������Ӧ�����ӷ���ʽ |
A | Fe2����NO | ϡ���� | 3Fe 2����NO |
B | Fe3����I����ClO�� | ����������Һ | Fe3����3OH��===Fe(OH)3�� |
C | Ba2����HCO | ����������Һ | HCO |
D | Al3����Cl����NO | ��������������Һ | Al3����3OH��===Al(OH)3�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�����ʡ�����и�����һ��ģ�⿼�Ի�ѧ�Ծ��������棩�� ���ͣ�ѡ����
��NAΪ�����ӵ�������ֵ������������ȷ����(�� ��)
A�����³�ѹ�£�1 mol�������еĵ�����ĿΪ4NA
B����CH3COONa��Һ��CH3COO������ĿΪNA����Na������Ŀ����NA
C��һ�������£�1 mol N2��3 mol H2��ϣ���Ӧת�Ƶĵ�����ĿΪ6NA
D����״���£�11.2 L�����к��еĻ�ѧ����ĿΪ9.5NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�����ʡ�����и�����һ��ģ�⿼�Ի�ѧ�Ծ��������棩�� ���ͣ�ѡ����
������ڻ�ѧѧ�Ƶķ�չ������Ҫ�����á����з������������(�� ��)
A�����ݴ������е�Ԫ����ɣ����������Ϊ���ʺͻ�����
B��������Һ��������ǿ����������ʷ�Ϊǿ����ʡ��������
C�������Ƿ���ж����ЧӦ������ɢϵ��Ϊ��Һ����Һ�ͽ���
D�����ݷ�Ӧ�е������仯������ѧ��Ӧ��Ϊ�����ϡ��ֽ⡢���ֽ⡢�û�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�����ʡ������һ���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
����ʵ��������¹ʴ����������( )
A��ʹ��ˮ���¶ȼƲ����ձ���ˮԡ�¶�ʱ����������ˮ�����õιܽ�ˮ����������ˮ���Сƿ�У����Ƶ��¶ȼƲ���װ����۵Ĺ��ƿ��
B�����Թܼд��Թܵ��������ϼ�ס���Թܿ�Լ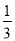 �����ֳ��Թܼг���ĩ�ˣ����м���
�����ֳ��Թܼг���ĩ�ˣ����м���
C���Ʊ���������ʱ�����Ҵ����������μ��뵽Ũ������
D���Ѳ����ܲ���������ʱ���ú��֣�������ˮʪ��IJ����ܲ���ˣ���������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�����ʡ������һ���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
��ȥ�����������������ʵķ�������ȷ����( )
A����ȥFeCl2��Һ�л��е�FeCl3������������ۣ�����
B����ȥCO2�л��е�HCl���ñ���NaHCO3��Һϴ��
C����ȥBaCO3�����л��е�BaSO4��������������ᡢ���ˡ�ϴ��
D����ȥ��Ȳ�л��е�����H2S��PH3��ͨ��CuSO4��Һ��ϴ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�����ʡ�߶���ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
�л���CH3-CH=CH-Cl�ܷ����ķ�Ӧ�� ( )
��ȡ����Ӧ���ڼӳɷ�Ӧ������ȥ��Ӧ����ʹ��ˮ��ɫ����ʹ����KMnO4��Һ��ɫ������AgNO3��Һ���ɰ�ɫ�������߾ۺϷ�Ӧ
A�����Ϸ�Ӧ���ɷ��� B��ֻ�Т��ܷ���
C��ֻ�Т߲��ܷ��� D��ֻ�Тڲ��ܷ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�����ʡ��������У�߶���ѧ������������ѧ�Ծ��������棩 ���ͣ�ѡ����
�����й������У�������ǣ� ��
A�����ʯ����״�ṹ�У��ɹ��ۼ��γɵ���С̼������6��̼ԭ��
B�������Ӿ����У���֮��ͨ�����ۼ����
C���Ҽ����Ե������ڣ��м����ɵ�������
D��H2O�ķֽ��¶ȼ��е㶼��H2S�ߵö����ʵ���ɶ������֪ʶ����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com