
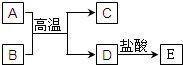
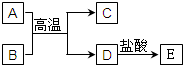
 ��ͼ��A��E��Ϊ��ѧ��ѧ�г��������ʣ�����֮��������ת����ϵ������A��DΪ�������ʣ�C�����������BΪ����ɫ��ĩ����Ӧ���������ɵ�ˮ��������������ȥ��
��ͼ��A��E��Ϊ��ѧ��ѧ�г��������ʣ�����֮��������ת����ϵ������A��DΪ�������ʣ�C�����������BΪ����ɫ��ĩ����Ӧ���������ɵ�ˮ��������������ȥ��

 ������ȫ�̼����ĩ���100��ϵ�д�
������ȫ�̼����ĩ���100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
![]() ��֪A~E��Ϊ��ѧ��ѧ�������ʣ�ת����ϵ��ͼ������A��B��C��E�����γ�ԭ�Ӿ��壬B��EΪ�ǽ������ʣ�C������β���е���Ⱦ��֮һ��
��֪A~E��Ϊ��ѧ��ѧ�������ʣ�ת����ϵ��ͼ������A��B��C��E�����γ�ԭ�Ӿ��壬B��EΪ�ǽ������ʣ�C������β���е���Ⱦ��֮һ��
(1)��Ӧ���У��������ͻ�ԭ���������ȣ� ��
(2)B��D��E�γ�ԭ�Ӿ�����۵��ɸߵ��͵�˳���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��ͼ��A��E��Ϊ��ѧ��ѧ�г��������ʣ�����֮��������ת����ϵ������A��DΪ�������ʣ�C�����������BΪ����ɫ��ĩ����Ӧ���������ɵ�ˮ��������������ȥ��
��ͼ��A��E��Ϊ��ѧ��ѧ�г��������ʣ�����֮��������ת����ϵ������A��DΪ�������ʣ�C�����������BΪ����ɫ��ĩ����Ӧ���������ɵ�ˮ��������������ȥ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꽭��ʡ�γ��н������ϸԸ���ѧ�߶����ϣ����л�ѧ�Ծ��������棩 ���ͣ������

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com