
ʵ������Ҫ����0.50 mol/L NaCl��Һ480 mL����ʹ��NaCl�������ƣ������в������������ʵ������֣���ʹ��������������
��.��1��ѡ����������ɱ�ʵ��������������У�������ƽ(��ȷ��0.1 g)��ҩ�ס��ձ�����������__________��_________________�Լ�����������Ƭ��ֽ��
��2�����㡣���Ƹ���Һ��ȡNaCl����________ g��
��3��������
����ƽ��ƽ�� �ڳ����� �۳�����ϣ���ҩƷ�����ձ��С�
��4���ܽ⡢��ȴ���ò�ʵ������Ҫʹ�ò�������Ŀ����___________________��
��5��ת�ơ�ϴ�ӡ���ת��ʱӦʹ��________��������Ҫϴ���ձ�2��3����Ϊ��_______________________��
��6�����ݡ�ҡ�ȡ�
��7������õ���Һ����һ��ʱ�����ָ�����Լ�ƿ�У������ñ�ǩ��ע�����Ƶ�ʱ�䡢��Һ���Ƽ�Ũ�ȡ�
��8�������ƹ����У�ijѧ���۲춨��ʱҺ�������ͼ��ʾ��������Һ��Ũ�Ȼ�______(�ƫ�ߡ�����ƫ�͡�����Ӱ�족)��
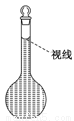
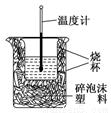
��ʵ����������ͼװ�ý����к��ȵIJⶨ����ش��������⣺
��1����ͼ��������δ������������________________��________________��
��2���ڲ�����ȷ��ǰ���£�����к��Ȳⶨȷ�ԵĹؼ���_________________��
��3������0.5 mol/L��������NaOH�������ʵ�飬��ʵ���в�õġ��к��ȡ���ֵ��________(�ƫ����ƫС���������䡱)��ԭ����_______________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ��������������ʵ����ѧ�߶������л�ѧ���������棩 ���ͣ�ѡ����
��25��ʱ����Ũ��Ϊ0.1000 mol��L��1��NaOH��Һ�ζ�20.00 mLŨ�Ⱦ�Ϊ0.1000 mol��L��1��������HX��HY��HZ�ζ�������ͼ��ʾ������˵����ȷ����

A������ͬ�¶��£�ͬŨ�ȵ���������Һ�ĵ�������˳��HZ��HY��HX
B�����ݵζ����ߣ��ɵ�Ka(HY)��10��6
C��������HX��HY��Һ�������Ϻ���NaOH��Һ�ζ���HXǡ����ȫ��Ӧʱ��
c(X��)��c(Y��)��c(OH��)��c(Na+)��c(H��)
D��HY��HZ��ϣ��ﵽƽ��ʱ��c(H��)��c(Y��)��c(Z��)��c(OH��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�꽭���߰���ѧ��һ�����л�ѧ�����ص�ࣩ�������棩 ���ͣ�ѡ����
������������Һ���ܴ����������( )
A��Cl����NO��Fe3����Na�� B��Ag����NO3-��Cl����K��
C��K����Ba2����OH����SO42- D��Cu2����NH4+��Br����OH��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�꺣��ʡ��һ�ϵ������¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
�������ӷ���ʽ�У���ȷ����
A���������ᷴӦ��2Fe+6H+=2Fe3++3H2��
B���Ȼ�����Һ������Ӧ��Fe3++Fe = 2Fe2+
C������������ϡ���ᷴӦ��FeO+2H+=Fe3++H2O
D���Ȼ�����Һ��ͭ��Ӧ��2Fe3++Cu=2Fe2++Cu2+
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ��㶫տ����һ��ѧ��һ�ϵڶ��ο��Ի�ѧ���������棩 ���ͣ�ѡ����
����������Һ�������ͺ��Ȼ�����Һ ��39%���Ҵ���Һ ���Ȼ��ƺ͵������ˮ��Һ���������ϸ����Һ����ȷ���������ǣ� ��
A����Һ����ȡ������
B����ȡ������Һ
C����Һ��������ȡ
D��������ȡ����Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ��㶫ʡ��һ�����в��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
����������ȷ����
A��1 mol H2��������������Է����������
B��CH4��Ħ������Ϊ16g
C��3.01��1023��SO2���ӵ�����Ϊ32g
D��Ħ�������ʵ����ĵ�λ������Ϊ n
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ��ӱ���ˮ������ѧ�߶����¿�����ѧ�Ծ��������棩 ���ͣ�ѡ����
��a��b��c��d�Ŀ����Ƭ������ϡ�����У��õ�����������������ɸ���ԭ��ء���a��b����ʱ��aΪ������c��d����ʱ�� d���������ݳ���a��c����ʱ��a�����b��d����ʱ��bΪ�������������ֽ����Ļ�������ǿ������˳��Ϊ�� ��
A��a��b��c��d B��a��c��b��d C��a��c��d��b D��b��d��a��c
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016��ɽ��ʦ���и�����ѧ�ڵڶ���ģ�⿼�Ի�ѧ�Ծ��������棩 ���ͣ������
����Z��һ�ֳ����İ뵼����ϣ�����Xͨ������ͼ��ʾ��·���Ʊ�������XΪZ�������YΪ�⻯����ӽṹ��������ƣ��ش��������⣻
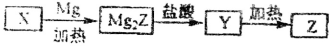
��1������X������ѧ��Ӧ������_______(д����)����X�Ʊ�Mg2Z�Ļ�ѧ����ʽΪ___ __��
��2����Mg2Z����Y�Ļ�ѧ��Ӧ����ʽΪ___________________��Y���ӵĵ���ʽΪ________________
��3��Z��X�л�ѧ�������Ͷ���_________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ���㽭ʡ����ʮУ������߶���ѧ�����в��Ի�ѧ�Ծ��������棩 ���ͣ������
����������������OH�������·���ˮ�ⷴӦ��
O2NC6H4COOC2H5��OH�� O2NC6H4COO����C2H5OH
O2NC6H4COO����C2H5OH
���ַ�Ӧ��ij�ʼŨ�Ⱦ�Ϊ0.050 mol��L��1��15 ��ʱ���O2NC6H4COOC2H5��Ũ��c��ʱ��仯�����������ʾ���ش��������⣺
t/s | 0 | 120 | 180 | 240 | 330 | 530 | 600 | 700 | 800 |
c/mol��L��1 | 0 | 0.036 | 0.030 | 0.026 | 0.022 | 0.017 | 0.016 | 0.015 | 0.015 |
��1������÷�Ӧ��120��180 s���ƽ����Ӧ����_________���Ƚ�120��180 s��180��240 s �����ƽ����Ӧ���ʵĴ�С��ǰ��_________���ߣ����������������____________________��
��2������15 ��ʱ�÷�Ӧ��ƽ�ⳣ��_____________��
(3)Ϊ���O2NC6H4COOC2H5��ƽ��ת���ʣ������ʵ����Ʒ�Ӧ�¶��⣬���ɲ�ȡ�Ĵ�ʩ��________��
A.����O2NC6H4COOC2H5 B.����OH����Ũ��
C.��ȥ���� D.�����ʵ��Ĵ���
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com