
����Ŀ����һ���¶��£������������ǿ��淴ӦA(g)��3B(g)![]() 2C(g)�ﵽƽ��״̬��־���ǣ� ��
2C(g)�ﵽƽ��״̬��־���ǣ� ��
��C���ɵ�������C�ֽ��������ȣ��ڵ�λʱ��������a mol A��ͬʱ����3a mol B����A��B��C��Ũ�Ȳ��ٱ仯����A��B��C��ѹǿ���ٱ仯���ݻ���������ѹǿ���ٱ仯�������������ʵ������ٱ仯���ߵ�λʱ��������a mol A��ͬʱ����3a mol B����A��B��C�ķ�����֮��Ϊ1��3��2
A.�ڢ�B.�٢�C.�ڢ�D.�ۢ�
���𰸡�A
��������
��C���ɵ�������C�ֽ��������ȣ���Ӧ��ƽ��״̬��
�ڵ�λʱ��������amolA��ͬʱ����3amolB����Ӧ������ͬ����һ����ƽ��״̬��
��A��B��C��Ũ�Ȳ��ٱ仯����Ӧ��ƽ��״̬��
��A��B��C��ѹǿ���ٱ仯����Ũ�Ȳ��䣬��Ӧ��ƽ��״̬��
����Ϊ��Ӧǰ��������������ȣ����Ի���������ѹǿ���ٱ仯����Ӧ��ƽ��״̬��
�����������ʵ������ٱ仯����Ӧ��ƽ��״̬��
�ߵ�λʱ��������amolA��ͬʱ����3amolB����Ӧ�����෴����Ӧ��ƽ��״̬��
��A��B��C�ķ�����֮��Ϊ1��3��2����Ӧ��һ����ƽ��״̬��
�ۺ����Ϸ�������һ����ƽ��״̬���Ǣڢࣻ��ѡA��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ݹ����ŵIJ�ͬ�������л�����з��ࡣ
��CH3CH2OH��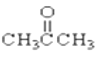 ��CH3CH2Br��
��CH3CH2Br��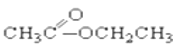 ��
��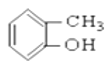 ��
��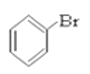 ��
��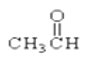 ��
��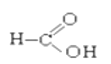 ��
��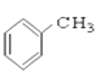 ��
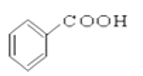
��
��1����������_____________________________________________________
��2��±������_____________________________________________________
��3������_________________________________________________________
��4���ӣ�_________________________________________________________
��5��ȩ��__________________________________________________________
��6��ͪ��__________________________________________________________
��7�����_________________________________________________________
��8������__________________________________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������(��Ԫ���ᣬ����ʽΪH2C2O4)�鲼����Ȼ�磬�������е�ֲ�ﶼ���в����(CaC2O4)��
(1) ������(C6H12O6)��HNO3��Ӧ�����ɲ����NO���仯ѧ����ʽΪ________��
(2) �൱һ��������ʯ����Ҫ�ɷ���CaC2O4����ij��ÿ��������Ϊ1.4 L����0.10 g Ca2��������Һ��c(C2O42-)>________mol��L��1ʱ�����γ�CaC2O4������[��֪Ksp(CaC2O4)��2.3��10��9]
(3) �ⶨij���ᾧ��(H2C2O4��xH2O)��ɵ�ʵ�����£�
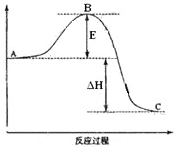
����1��ȷ��ȡ0. 550 8 g�ڱ����������(�ṹ��ʽΪ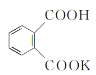 )����ƿ�У�������ˮ�ܽ⣬�Է�̪��ָʾ������NaOH��Һ�ζ����յ㣬����NaOH��Һ�����Ϊ22.50 mL��
)����ƿ�У�������ˮ�ܽ⣬�Է�̪��ָʾ������NaOH��Һ�ζ����յ㣬����NaOH��Һ�����Ϊ22.50 mL��
����2��ȷ��ȡ0.151 2 g���ᾧ������ƿ�У�������ˮ�ܽ⣬�Է�̪��ָʾ�����ò���1������NaOH��Һ�ζ����յ�(H2C2O4��2NaOH===Na2C2O4��2H2O)������NaOH��Һ�����Ϊ20.00 mL��
��������1����Ŀ����____________________________________��
�ڼ���x��ֵ(д���������)__________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����з����п���֤���Ҵ��������ǻ��ϵ���ԭ������������������ԭ�����Բ�ͬ���ǣ� ��
A.1mol�Ҵ���ȫȼ������3mol![]()
B.�Ҵ�����������
C.1mol�Ҵ���������![]() ���õ�0.5mol
���õ�0.5mol![]()
D.1mol �Ҵ��ڴ��������¿���������Ӧ����1mol��ȩ.
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʵ�����������ͽ��۾���ȷ����
ѡ�� | ʵ����� | ���� | ���� |
A | ��˫��ˮ�ζ�KI-������Һ | ��Һ���� | �ﵽ�ζ��յ� |
B | ��ʳ�üӵ����м���ʳ��KI��Һ���ټ���CCl4������ | �²���Ϻ�ɫ | ��ʳ�üӵ����к���KIO3 |
C | ��ʪ��ĵ��۵⻯����ֽ����NO2�������� | ��ֽ���� | ������Ϊ������ |
D |
| ����Թ���dz��ɫ���� | �л����к�����ԭ�� |
A. AB. BC. CD. D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ͼ��ʾʵ��װ��(�г���������ȥ)̽��ͭ˿�����Ũ����ķ�Ӧ������ʵ�鲻��������( )

A. �����ƶ�����ͭ˿�ɿ���SO2����
B. ����ѡ��Ʒ����Һ��֤SO2������
C. ����ѡ��NaOH��Һ���ն����SO2
D. Ϊȷ��CuSO4���ɣ������м�ˮ���۲���ɫ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ӵĹ�ҵ��ˮ�Ĵ���������ͼ��ʾ��

��1��������ͼ�豸���н��е��Dz���________(��д��������)��ʵ��������һ������������_______(����������)���С�
�����豸�������豸��������A��______________(�ѧʽ����ͬ)�����豸�������豸��������B��___________��
�����豸���з�����Ӧ�Ļ�ѧ����ʽΪ_____________________��
�����豸���У�����B��ˮ��Һ��CaO��Ӧ������NaOH��H2O��______��ͨ�� _________________(���������)����������ʹ��������롣
��ͼ�У���ѭ��ʹ�õ�������_______��_____________C6H6��CaO��
��2��Ϊ�˷�ֹˮԴ��Ⱦ���ü����������Եķ�������ij�����ŷŵ���ˮ�����ޱ��ӣ��˷�����____________��
�ӷ�ˮ�л��ձ��ӵķ����Ǣ����л��ܼ���ȡ��Һ�еı��ӣ��ڼ���ij��ҩƷ��ˮ��Һʹ�������л��ܼ����룻�ۼ���ij�������������ӡ���д�������������ķ�Ӧ����ʽ��_________________________��
��3��Ϊ�ⶨ��ˮ�б��ӵĺ�����ȡ�˷�ˮ100 mL�������м���Ũ��ˮ�����ٲ�������Ϊֹ���õ�����0.331 g����˷�ˮ�б��ӵĺ���____________(mg��L��1)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ش��������⣺
��1����֪2mol����ȼ������Һ̬ˮʱ�ų�572kJ����������Ӧ����ʽ��2H2(g)+O2(g)=2H2O(l)��
�ٸ÷�Ӧ�������������ܺ�__(��������������С��������������)��Ӧ�������ܺ͡�
����2mol������ȫȼ������ˮ��������ų�������___(��������������С��������������)572 kJ��
��2��2.3g�л���C2H6O��һ������������ϵ�ȼ��ǡ����ȫȼ�գ�����CO2��Һ̬ˮ�����ų�68.35kJ��������÷�Ӧ���Ȼ�ѧ����ʽ��___��
��3��FeS2���ղ�����SO2�����������ᡣ��֪25�桢101kPaʱ��
2SO2(g)+O2(g)![]() 2SO3(g) ��H1=-197 kJ��mol-1
2SO3(g) ��H1=-197 kJ��mol-1
H2O(g)=H2O(l) ��H2=-44kJ��mol-1
2SO2(g)+O2(g)+2H2O(g)=2H2SO4(l) ��H3=-545kJ��mol-1
��SO3(g)��H2O(l)��Ӧ����H2SO4(l)���Ȼ�ѧ����ʽ��___��
��4��2SO2(g)+O2(g)![]() 2SO3(g)��Ӧ���̵������仯��ͼ��ʾ���÷�Ӧͨ����V2O5����������V2O5��ʹͼ��B��___(����������������������)����H___(���������������С������������)��
2SO3(g)��Ӧ���̵������仯��ͼ��ʾ���÷�Ӧͨ����V2O5����������V2O5��ʹͼ��B��___(����������������������)����H___(���������������С������������)��
��5���к��Ȳⶨ��ʵ���У��õ��IJ����������ձ����¶ȼơ�___����Ͳ����ȡ��Ӧ��ʱ��ȡ50 mL 0.50 mol��L-1�����ᣬ���������Լ���___(�����)��
A.50 mL0.50mol��L-1NaOH��Һ
B.50 mL0.55mol��L-1NaOH��Һ
C.1.0gNaOH����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�л�������Ľṹ��ʽΪ��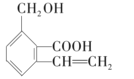
��1����д�����л�����еĹ��������ƣ�
��___________����___________����____________��
��2�����л�������___________________________����ѡ����ĸ����
��������� �ڶ�������л��� �۷����� ������������ �ݴ������ ���㻯���� ��ϩ��
A.�٢ڢۢ� B.�٢ڢܢ� C.�ڢܢݢ� D.�ڢݢޢ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com