
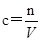 ��֪�����ձ����ܽ����ʣ�����ʱ��������������Һ�������ʵ��������٣�Ũ��ƫ�ͣ�δ��ϴ���ձ��ڱڵ���Һת��������ƿ��ͬ���������������٣�Ũ��ƫ�ͣ�����ƿ���������ҺҺ��δ���̶��߱�ֹͣ��ˮ��������ƿ����Һ������٣�Ũ��ƫ�ߣ�����õ���Һ������ƿת�Ƶ�����ྻ���Լ�ƿ��ʱ��������������Ũ�Ȳ��䣻���ձ�����Һת�Ƶ�����ƿ֮ǰ������ƿ������������ˮ������Ӱ��ʵ����������ƿ��Һ�潫�ﵽ�̶���ʱ�����ӿ̶��ߺ�Һ�棬������ƿ����Һ������٣�Ũ��ƫ�ߡ�
��֪�����ձ����ܽ����ʣ�����ʱ��������������Һ�������ʵ��������٣�Ũ��ƫ�ͣ�δ��ϴ���ձ��ڱڵ���Һת��������ƿ��ͬ���������������٣�Ũ��ƫ�ͣ�����ƿ���������ҺҺ��δ���̶��߱�ֹͣ��ˮ��������ƿ����Һ������٣�Ũ��ƫ�ߣ�����õ���Һ������ƿת�Ƶ�����ྻ���Լ�ƿ��ʱ��������������Ũ�Ȳ��䣻���ձ�����Һת�Ƶ�����ƿ֮ǰ������ƿ������������ˮ������Ӱ��ʵ����������ƿ��Һ�潫�ﵽ�̶���ʱ�����ӿ̶��ߺ�Һ�棬������ƿ����Һ������٣�Ũ��ƫ�ߡ�


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
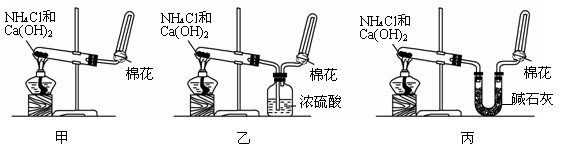
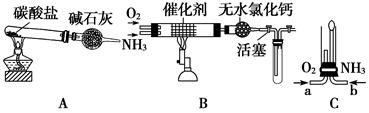
�鿴�𰸺ͽ���>>
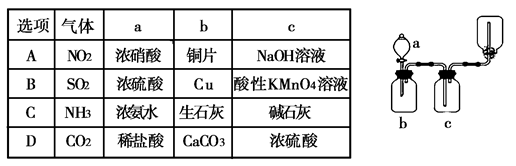
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
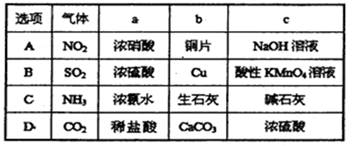
�鿴�𰸺ͽ���>>
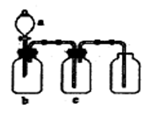
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
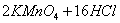 (Ũ)="==="
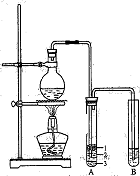
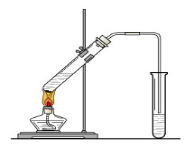
(Ũ)="===" 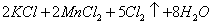 ����ʵ���ҿ��ö������̹���������ع����Ũ���ᷴӦ��ȡ�������ɹ�ѡ�õķ���װ������ͼ��
����ʵ���ҿ��ö������̹���������ع����Ũ���ᷴӦ��ȡ�������ɹ�ѡ�õķ���װ������ͼ��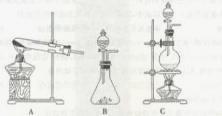
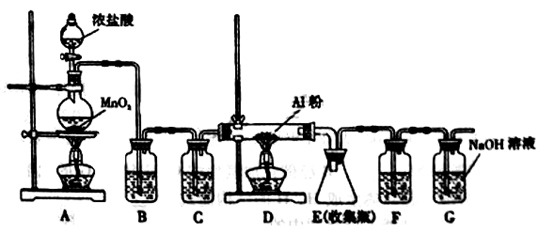
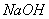 ��Һ����������ֹ��Ⱦ,д���÷�Ӧ�����ӷ���ʽ ��
��Һ����������ֹ��Ⱦ,д���÷�Ӧ�����ӷ���ʽ ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| A��5mL�������� | B��10mL | C��25mL | D��50mL |

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com