
ЁОЬтФПЁПЃЈ1ЃЉ4.9 g H2SO4КЌ________________ИіH2SO4ЗжзгЃЌФмКЭ__________mol NaOHЭъШЋЗДгІЁЃ
ЃЈ2ЃЉКЌгаЯрЭЌЬМдзгЪ§ЕФCOКЭCO2ЃЌЦфжЪСПБШЮЊ________________ЁЃ
ЃЈ3ЃЉдкЭЌЮТЭЌбЙЯТЃЌЕШжЪСПЕФCOКЭNOЃЌЦфЬхЛ§жЎБШЮЊ___________ЁЃ
ЃЈ4ЃЉБъзМзДПіЯТгаЂй44.8 L CH4ЃЌЂк9.03ЁС1023ИіNH3ЗжзгЃЌЂл85 g H2SШ§жжЦјЬхЃЌЖдетШ§жжЦјЬхЕФЮяжЪЕФСПДгДѓЕНаЁЕФЫГађЪЧ________________ (ЬюађКХ)ЁЃ
ЃЈ5ЃЉдквЛЖЈЕФЮТЖШКЭбЙЧПЯТЃЌ1ЬхЛ§ЦјЬхX2Ињ3ЬхЛ§ЦјЬхY2ЛЏКЯЩњГЩ2ЬхЛ§ЛЏКЯЮяCЃЌдђИУЛЏКЯЮяЕФЛЏбЇЪНЪЧ____________ЁЃ
ЁОД№АИЁП3.01ЁС1022 (Лђ0.05NA) 0.1 7ЁУ11 15ЁУ14 ЂлЁЂЂйЁЂЂк XY3 (ЛђY3X)
ЁОНтЮіЁП
ЃЈ1ЃЉ4.9 g H2SO4ЕФЮяжЪЕФСПЮЊ4.9gЁТ98g/mol=0.05molЃЌдђКЌгаСђЫсЗжзгИіЪ§ЮЊ0.05 NAЃЌгЩЗДгІ2NaOH+H2SO4=Na2SO4+2H2OЃЌПЩжЊЯћКФNaOHЕФЮяжЪЕФСПЮЊ0.05molЁС2=0.1molЃЌЙЪД№АИЮЊЃК3.01ЁС1022 (Лђ0.05NA)ЁЂ0.1ЃЛ
ЃЈ2ЃЉКЌгаЯрЭЌЬМдзгЪ§ЕФCOКЭCO2ЃЌЦфЮяжЪЕФСПЯрЕШЃЌдђЦфжЪСПБШЮЊ28ЃК44=7:11ЃЌЙЪД№АИЮЊ7:11ЃЛ
ЃЈ3ЃЉдкЭЌЮТЭЌбЙЯТЃЌЕШжЪСПЕФCOКЭNOЃЌЦфЮяжЪЕФСПжЎБШЮЊ![]() =15:14ЃЌЦфЬхЛ§жЎБШЕШгкЦфЮяжЪЕФСПжЎБШЃЌЙЪД№АИЮЊ15:14ЃЛ
=15:14ЃЌЦфЬхЛ§жЎБШЕШгкЦфЮяжЪЕФСПжЎБШЃЌЙЪД№АИЮЊ15:14ЃЛ
ЃЈ4ЃЉБъзМзДПіЯТЂй44.8 L CH4ЃЌЂк9.03ЁС1023ИіNH3ЗжзгЃЌЂл85 g H2SШ§жжЦјЬхЃЌетШ§жжЦјЬхЕФЮяжЪЕФСПЗжБ№ЮЊ![]() =2molЁЂ
=2molЁЂ![]() =1.5molЁЂ
=1.5molЁЂ![]() =2.5molЃЌЙЪД№АИЮЊЃКЂлЁЂЂйЁЂЂкЃЛ
=2.5molЃЌЙЪД№АИЮЊЃКЂлЁЂЂйЁЂЂкЃЛ
ЃЈ5ЃЉИљОнАЂЗќМгЕТТоЖЈТЩЃЌЦјЬхЕФЬхЛ§жЎБШЕШгкЮяжЪЕФСПжЎБШЃЌЕШгкЛЏбЇЗНГЬЪНЕФМЦСПЪ§жЎБШЃЌПЩЕУЃКX2+3Y2=2CЃЌИљОнЛЏбЇЗДгІЧАКѓдзгжжРрКЭЪ§ФПЯрЕШЃЌПЩЕУЩњГЩЮяЗжзгКЌ1ИіXдзгЃЌ3ИіYдзгЃЌЛЏбЇЪНЮЊXY3ЃЌЙЪД№АИЮЊЃКXY3 (ЛђY3X)ЁЃ


 дФЖСПьГЕЯЕСаД№АИ
дФЖСПьГЕЯЕСаД№АИ
| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПдкСНИіУмБеШнЦїжаЃЌЗжБ№ГфгажЪСПЯрЭЌЕФМзЁЂввСНжжЦјЬхЃЌШєСНШнЦїЕФЮТЖШКЭбЙЧПОљЯрЭЌЃЌЧвМзЕФУмЖШДѓгкввЕФУмЖШЁЃдђЯТСаЫЕЗЈе§ШЗЕФЪЧЃЈ ЃЉ
A. ЮяжЪЕФСПЃКМзЃОвв B. ЦјЬхЬхЛ§ЃКМзЃОвв
C. ЦјЬхФІЖћЬхЛ§ЃКМзЃОвв D. ЯрЖдЗжзгжЪСПЃКМзЃОвв
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПвбжЊЃК2CO(g)ЃЋO2(g)===2CO2(g)ЁЁІЄHЃНЃ566 kJЁЄmolЃ1
Na2O2(s)ЃЋCO2(g)===Na2CO3(s)ЃЋ![]() O2(g)ЁЁІЄHЃНЃ266 kJЁЄmolЃ1
O2(g)ЁЁІЄHЃНЃ266 kJЁЄmolЃ1
ИљОнвдЩЯШШЛЏбЇЗНГЬЪНХаЖЯЃЌЯТСаЫЕЗЈе§ШЗЕФЪЧ(ЁЁЁЁ)
A. COЕФШМЩеШШЮЊ283 kJ
B. гвЭМПЩБэЪОгЩCOЩњГЩCO2ЕФЗДгІЙ§ГЬКЭФмСПЙиЯЕ

C. 2Na2O2(s)ЃЋ2CO2(s)===2Na2CO3(s)ЃЋO2(g)ЁЁІЄH > Ѓ532 kJЁЄmolЃ1
D. CO(g)гыNa2O2(s)ЗДгІЗХГі549 kJШШСПЪБЃЌЕчзгзЊвЦЪ§ЮЊ6.02ЁС1023
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПWЁЂXЁЂYЁЂZЪЧЫФжжГЃМћЕФЖЬжмЦкдЊЫи,ЦфдзгАыОЖЫцдзгађЪ§БфЛЏШчЭМЫљЪО.вбжЊWЕФвЛжжКЫЫиЕФжЪСПЪ§ЮЊ18,жазгЪ§ЮЊ10;XКЭNeдзгЕФКЫЭтЕчзгЪ§ЯрВю1;YЕФЕЅжЪЪЧвЛжжГЃМћЕФАыЕМЬхВФСЯ;ZЕФЗЧН№ЪєаддкЭЌжмЦкжїзхдЊЫижазюЧП.ЯТСаБэЪіжае§ШЗЕФЪЧЃЈ ЃЉ
A. XЮЛгкдЊЫижмЦкБэжаЕкЖўжмЦкЕкIAзх
B. XЕФЕЅжЪКЭYЕФЕЅжЪЯрБШ,ШлЕуНЯИпЕФЪЧX
C. ZЕФбѕЛЏЮяЕФЫЎЛЏЮявЛЖЈЪЧЧПЫс
D. WЁЂXПЩаЮГЩX2WЁЂX2W2РызгЛЏКЯЮяЃЌЧввѕбєРызгИіЪ§БШОљЮЊ1:2
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПТШЛЏбЧэПЃЈSOCl2ЃЉЪЧвЛжжвКЬЌЛЏКЯЮяЃЌЗаЕуЮЊ77ЁцЃЌдкХЉвЉЁЂжЦвЉаавЕжагУЭОЙуЗКЃЎSOCl2гіЫЎОчСвЗДгІЃЌвКУцЩЯВњЩњАзЮэЃЌВЂДјгаДЬМЄадЦјЮЖЕФЦјЬхВњЩњЃЎЪЕбщЪвКЯГЩдРэЃКSO2+Cl2+SCl2ЈT2SOCl2ЃЌВПЗжзАжУШчЭМЫљЪОЃЌЛиД№вдЯТЮЪЬтЃК

ЃЈ1ЃЉвЧЦїcЕФУћГЦЪЧ________
ЃЈ2ЃЉЪЕбщЪвжЦCl2ЕФЛЏбЇЗНГЬЪНЮЊ________________________
ЃЈ3ЃЉЯТСаЫФжжжЦБИ SO2ЕФЗНАИжазюМббЁдёЪЧ_______
ЗНАИ | Мз | вв | Бћ | ЖЁ |
ЗЂЩњзАжУ |
|
|
|
|
ЫљбЁЪдМС |
|
|
|
|
ЃЈ4ЃЉdЕФащЯпПђФквўКЌСНИізАжУЃЌАДЦјСїЗНЯђЫГађетСНИізАжУЕФзїгУЗжБ№ЪЧ_______________ЁЃ
ЃЈ5ЃЉЪЕбщНсЪјКѓ,НЋШ§ОБЩеЦПжаЛьКЯЮяЗжРыПЊЕФЗНЗЈЪЧ _________(вбжЊSCl2ЕФЗаЕуЮЊ50Ёц)
ЃЈ6ЃЉЪдЩшМЦвЛИіМђЕЅЪЕбщЗНАИбщжЄH2SO3ЫсадЧПгкH2CO3ЃЈМђвЊЫЕУїЪЕбщВНжшЁЂЯжЯѓКЭНсТлЃЉ____________________________________________ЁЃвЧЦїздбЁЁЃ
ЯобЁЕФЪдМСЃКSO2ЁЂNaHCO3ЁЂЫсадKMnO4ЁЂNaHSO3ЁЂеєСѓЫЎЁЂБЅКЭЪЏЛвЫЎЁЂЦЗКьШмвКЁЂpHЪджНЁЃ
ЃЈ7ЃЉЮЊВтЖЈФГЙЄГЇЕФПеЦјжаЖўбѕЛЏСђКЌСПЃЌЬНОПаЁзщНјааШчЯТЪЕбщЃКдкЪдЙмжаМгШывЛЖЈСПЕФКЌЕтЃЈI2ЃЉ0.635mgЕФЕтШмвКЃЌдйМгШы2~3ЕЮЕэЗлШмвКЃЌЯђЪдЙмжаЭЈШыПеЦјЃЌЕБШмвКгЩРЖЩЋБфЮЊЮоЩЋЪБЧЁКУЭъШЋЗДгІЃЌЙВгУШЅПеЦјЬхЛ§ЮЊ500LЁЃЧыЭЈЙ§МЦЫуХаЖЯГіДЫПеЦјжаЖўбѕЛЏСђЕФХЈЖШ_____mg/m3ЁЃЃЈЛЏбЇЗНГЬЪНЮЊЃКSO2+I2+2H2O=H2SO4+2HIЃЉ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПШчЭМЫљЪОЃЌМзГиЕФзмЗДгІЪНЮЊЃК ![]() ЃЌЯТСаЙигкИУЕчГиЙЄзїЪБЕФЫЕЗЈе§ШЗЕФЪЧ
ЃЌЯТСаЙигкИУЕчГиЙЄзїЪБЕФЫЕЗЈе§ШЗЕФЪЧ![]()

A. ИУзАжУЙЄзїЪБЃЌAgЕчМЋЩЯгаЦјЬхЩњГЩ
B. МзГижаИКМЋЗДгІЮЊ![]()
C. МзГиКЭввГижаЕФШмвКЕФpHОљМѕаЁ
D. ЕБМзГижаЯћКФ![]()
![]() ЪБЃЌввГижаРэТлЩЯзюЖрВњЩњ
ЪБЃЌввГижаРэТлЩЯзюЖрВњЩњ![]() ЙЬЬх
ЙЬЬх
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁП
щФРЦМюдквНСЦЩЯГЃгУгкжЮСЦЧрЙтблЃЌЦфвЛжжКЯГЩТЗЯпШчЯТЃК
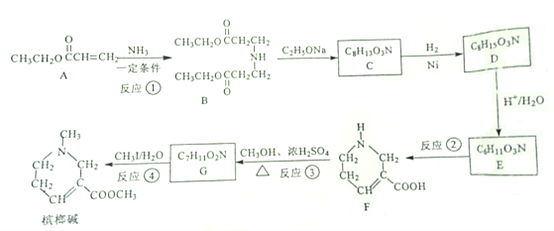 вбжЊЃК
вбжЊЃК

ЃЈ1ЃЉBжаКЌбѕЙйФмЭХЕФУћГЦЮЊ_________ЁЃ
ЃЈ2ЃЉЗДгІЂйЕФЗДгІРраЭЮЊ________ЁЃ
ЃЈ3ЃЉЗДгІЂлЕФЛЏбЇЗНГЬЪНЮЊ____________________ЁЃ
ЃЈ4ЃЉCЕФНсЙЙМђЪНЮЊ__________ЁЃ
ЃЈ5ЃЉЛЏКЯЮяXЪЧAЕФЭЌЗжвьЙЙЬхЃЌXМШФмгыЬМЫсЧтФЦШмвКЗДгІЗХГіCO2ЃЌ
гжФмЪЙфхЕФЫФТШЛЏЬМШмвКЭЪЩЋЁЃЦфКЫДХЙВеёЧтЦзЯдЪОга4жжВЛЭЌЛЏКЯЮяЛЗОГЕФЧтЃЌЗхУцЛ§БШЮЊ3:2:2:1ЃЌЪдаДГіЦфжавЛжжЗћКЯвЊЧѓЕФXЕФНсЙЙ
МђЪНЃК______________ЁЃ
ЃЈ6ЃЉвбжЊAдкЧтбѕЛЏФЦШмвКжаЫЎНтЕФВњЮяжЎвЛЪЧвЛжжаТаЭЙІФмИпЗжзгВФСЯЃЈPAANaЃЉЕФЕЅЬхЃЌаДГіЩњГЩPAANaЕФЛЏбЇЗНГЬЪН_________________ЁЃ
ЃЈ7ЃЉНсКЯБОЬтаХЯЂЃЌаДГівдввДМЮЊЦ№ЪМдСЯжЦБИЛЏКЯЮяCH3COCH2COOCH2CH3ЕФКЯГЩТЗЯпЃЈЮоЛњЪдМСШЮбЁЃЉ_________________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТСаБфЛЏВЛашвЊЦЦЛЕЛЏбЇМќЕФЪЧЃЈЁЁЁЁЃЉ
A. ТШЛЏяЇЪмШШЗжНт B. ИЩБљЦјЛЏ C. ЪГбЮШлЛЏ D. ТШЛЏЧтШмгкЫЎ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПФсВДН№ввѕЅЪЧвЛжжИпаЇЪГЦЗЗРИЏМСЃЌБЛЙуЗКгІгУгкНДгЭЁЂДзЕШЕїЮЖЦЗЁЂКцБКЪГЦЗвдМАЙћЪпБЃЯЪЕШСьгђЁЃЯТЭМЪЧвдБНЕШЮЊдСЯКЯГЩФсВДН№ввѕЅЕФКЯГЩТЗЯп:
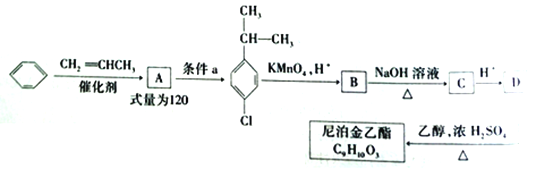
вбжЊ: 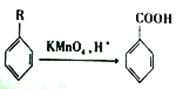 (RЮЊЬўЛљ)
(RЮЊЬўЛљ)
ЧыЛиД№гаЙиЮЪЬт:
ЃЈ1ЃЉгЩБНЩњГЩAЕФЗДгІРраЭЪЧ______ЃЌЬѕМўaжИЕФЪЧ_____ЃЌЛЏКЯЮяDжаЫљКЌЙйФмЭХЕФУћГЦЮЊ____________ЁЃ
ЃЈ2ЃЉаДГіBЁњC ЕФЛЏбЇЗНГЬЪН________________ЁЃ
ЃЈ3ЃЉФсВДН№ввѕЅЕФНсЙЙМђЪНЮЊ_________ЁЃ
ЃЈ4ЃЉЛЏКЯЮяDЕФЭЌЗжвьЙЙЬхжаКЌгаБНЛЗЧвФмЗЂЩњвјОЕЗДгІЕФга______жжЃЌЦфжаКЫДХЙВеёЧтЦзга4зщЗхЕФЭЌЗжвьЙЙЬхЕФНсЙЙМђЪНЮЊ_________(ШЮаДСНжж)ЁЃ
ЃЈ5ЃЉШєвдБНЮЊдСЯ( ЦфЫќдСЯздбЁ)КЯГЩзАБНМзЫсМзѕЅЃЌ ЂееЩЯЪіСїГЬЩшМЦаДГіКЯГЩТЗЯпЁЃ____________
ВщПДД№АИКЭНтЮі>>
ЙњМЪбЇаЃгХбЁ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com