
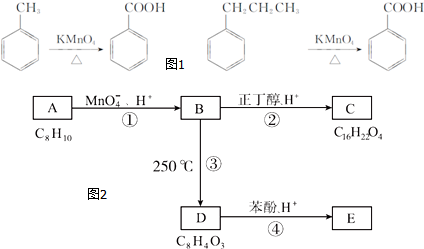
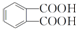 ��
��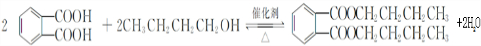 ���÷�Ӧ������Ϊȡ����Ӧ��
���÷�Ӧ������Ϊȡ����Ӧ��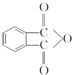 ����D���ʵĺ˴Ź�������ͼ�У������2��壬�����֮��Ϊ1��1��
����D���ʵĺ˴Ź�������ͼ�У������2��壬�����֮��Ϊ1��1��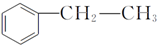 ��д�ṹ��ʽ����
��д�ṹ��ʽ���� ���� A�ķ���ʽΪCH8H10�����ڷ��㻯���ֻ������3��һ�廯�����AΪ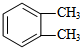 ��B�����ԣ���֪BΪ
��B�����ԣ���֪BΪ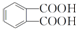 ��B������������������Ӧ����C�����C�ķ���ʽ��֪��C�Ľṹ��ʽΪ
��B������������������Ӧ����C�����C�ķ���ʽ��֪��C�Ľṹ��ʽΪ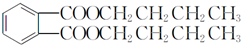 ��B���ȵõ�D�����D�ķ���ʽ��֪DΪ
��B���ȵõ�D�����D�ķ���ʽ��֪DΪ ��
��
��� �⣺A�ķ���ʽΪCH8H10�����ڷ��㻯���ֻ������3��һ�廯�����AΪ ��B�����ԣ���֪BΪ
��B�����ԣ���֪BΪ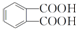 ��B������������������Ӧ����C�����C�ķ���ʽ��֪��C�Ľṹ��ʽΪ
��B������������������Ӧ����C�����C�ķ���ʽ��֪��C�Ľṹ��ʽΪ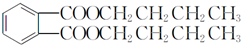 ��B���ȵõ�D�����D�ķ���ʽ��֪DΪ
��B���ȵõ�D�����D�ķ���ʽ��֪DΪ ��
��
��1��AΪ ������Ϊ�ڶ��ױ����ʴ�Ϊ���ڶ��ױ���
������Ϊ�ڶ��ױ����ʴ�Ϊ���ڶ��ױ���
��2��B�Ľṹ��ʽΪ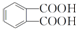 ���ʴ�Ϊ��
���ʴ�Ϊ��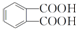 ��
��
��3����B����C�Ļ�ѧ����ʽΪ��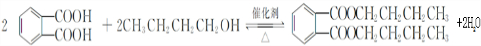 ���÷�Ӧ������Ϊȡ����Ӧ��
���÷�Ӧ������Ϊȡ����Ӧ��
�ʴ�Ϊ��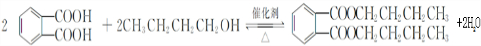 ��ȡ����
��ȡ����
��4��D�Ľṹ��ʽΪ ����D���ʵĺ˴Ź�������ͼ�У������2��壬�����֮��Ϊ 1��1��
����D���ʵĺ˴Ź�������ͼ�У������2��壬�����֮��Ϊ 1��1��
�ʴ�Ϊ�� ��2��1��1��
��2��1��1��
��5��A��ͬ���칹�������ڷ����廯������м���ױ����Զ��ױ����ұ�3�֣�����һ�ȴ���������������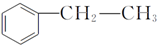 ��
��
�ʴ�Ϊ��3��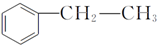 ��
��
���� ���⿼���л���ѧ�ƶϣ�ע������л������ʽ��������������չ�����������ת�����ѶȲ���


 �����Ļ���������人������ϵ�д�
�����Ļ���������人������ϵ�д� ���������ּ���ÿһ��ȫ�º�����ҵ��ϵ�д�
���������ּ���ÿһ��ȫ�º�����ҵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | c��PO43-����Na3PO4��Na2HPO4��NaH2PO4��H3PO4 | |
| B�� | c��CO32-������NH4��2CO3��Na2CO3��NaHCO3��NH4HCO3 | |
| C�� | c��NH4+������NH4��2SO4����NH4��2CO3��NH4HSO4��NH4Cl | |
| D�� | c��S2-����Na2S��NaHS��H2S |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 0.45mol/��L•min�� | B�� | 0.55mol/��L•min�� | C�� | 0.60mol/��L•min�� | D�� | 0.90mol/��L•min�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ������������һ�ֳ�Ӳ���ϣ����ɣ�2Mg3B2N4+3O2 $\frac{\underline{\;\;\;\;\;���£�����\;\;\;\;\;}}{���Ӽ�������}$6MgO+4BN+2N2�Ʊ���
������������һ�ֳ�Ӳ���ϣ����ɣ�2Mg3B2N4+3O2 $\frac{\underline{\;\;\;\;\;���£�����\;\;\;\;\;}}{���Ӽ�������}$6MgO+4BN+2N2�Ʊ����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ͨ������У�CH3COOH+NH3�TCH3COO-+NH4+ | |
| B�� | ��̼����þ��Һ�мӹ���ʯ��ˮ��Mg2++2HCO3-+Ca2++2OH-�TCaCO3��+2H2O+MgCO3�� | |
| C�� | ����ʯ��ˮ��ϡ���ᷴӦ��Ca��OH��2+2H+�TCa2++2H2O | |
| D�� | ϡ�������ͭƬ�ϣ�Cu+2H+�TCu2++H2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| W | X | Y | Z | |
| �ṹ������ | ����������Ӧ��ˮ����������̬�⻯�ﷴӦ�õ����ӻ����� | ��ɫ��Ӧ�ʻ�ɫ | ��ͬ��������Ԫ���γɵļ������У����Ӱ뾶��С | ��������������֮��Ϊ�� |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ú̿��������ʯ�;������ѻ����ѽ⻯���������ɻ�������Դ����Ҫ�Ļ���ԭ�� | |
| B�� | �������Ͷ����ص�Ǧ����ʹ��ʱ��ѹ���ȶ��������б��������͵��ȡ�������� | |
| C�� | �����Ʒ����Ҫ�ɷ��뽨������ɰ����ͬ | |
| D�� | ����10�ŷɴ�����̫���ܵ�ذ�ɽ�����ת��Ϊ���ܣ�����ת�����ϵ�����Ҳ�����Ʊ�����оƬ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

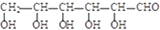 ��������������Ϊ
��������������Ϊ ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ƹ㡰��̼���á�����������������ŷ� | |
| B�� | ����̫���ܡ����ܺ����ܵ���Դ���滯ʯȼ�ϣ������ڽ�Լ��Դ���������� | |
| C�� | ���á���ɫ��ѧ�����գ�ʹԭ�Ͼ�����ת��Ϊ����Ҫ������ | |
| D�� | ��ͣ������ҵ��������ȾԴͷ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com