
����Ŀ��A��B��C�ǵ��ʣ�����A�ǽ������������ʼ��ת����ϵ��ͼ��
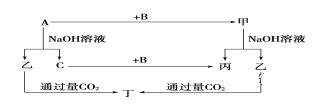
����ͼʾת����ϵ�ش�
(1)д���������ʵĻ�ѧʽ��A_______��B___________����__________����_______��
(2)д�����б仯�ķ���ʽ��
��A��NaOH��Һ��Ӧ�Ļ�ѧ����ʽ_______________________��
�ڼ���NaOH��Һ��Ӧ�����Һͱ������ӷ���ʽ_______________________��
(3)��һ������A���뵽NaOH��Һ�У�������C�ڱ�״���µ����Ϊ3.36 L�������ĵ�A�����ʵ���Ϊ________��ת�Ƶ��ӵ����ʵ���Ϊ________��
���𰸡�Al O2�� NaAlO2 Al(OH)3 2Al��2NaOH��2H2O===2NaAlO2��3H2�� Al2O3��2OH��===2AlO2����H2O 0.1 mol 0.3 mol
��������
A�ǽ������ʣ�A����NaOH��Һ��Ӧ��������������NaOH��Һ��Ӧ��ΪAl����AΪAl��Al��NaOH��Һ��Ӧ����NaAlO2��H2��C�ǵ��ʣ�CΪH2����ΪNaAlO2��B�ǵ��ʣ�Al+B���ף���+NaOH����+NaAlO2��H2+B��������BΪO2����ΪAl2O3����ΪH2O����ƫ��������Һ��ͨ�������̼����������������������Al(OH)3���ݴ��жϡ�
�������Ϸ�����֪AΪAl��BΪO2��CΪH2����ΪAl2O3����ΪNaAlO2����ΪH2O������Al(OH)3����
��1��A�Ļ�ѧʽΪAl��B�Ļ�ѧʽΪO2���ҵĻ�ѧʽΪNaAlO2�����Ļ�ѧʽΪAl(OH)3��
��2����Al��NaOH��Һ��Ӧ����ƫ�����ƺ���������Ӧ�Ļ�ѧ����ʽΪ2Al+2NaOH+2H2O��2NaAlO2+3H2����
��Al2O3��NaOH��Һ��Ӧ����ƫ�����ƺ�ˮ����Ӧ�����ӷ���ʽΪAl2O3��2OH����2AlO2����H2O��
��3��n��H2��=3.36L��22.4L/mol=0.15mol�����ݷ�Ӧ2Al+2NaOH+2H2O��2NaAlO2+3H2����֪n��Al��=2/3��0.15mol=0.1mol����Ӧ����1molAlת��3mol���ӣ���÷�Ӧ��ת�Ƶ������ʵ���Ϊ0.3mol��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����к�������NaCl��Na2SO4��Na2CO3����ɳ��SiO2�������ʵ�NaNO3���壬ѡ���ʵ����Լ���ȥ���ʣ��õ�������NaNO3���壬ʵ����������ͼ��ʾ��
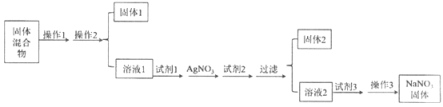
��1������1������3�����Ʒֱ���_________��_________��
��2���Լ�1���Լ�2�ֱ���_________��_________������2�г��˺���AgCl������_________����ȫ����
��3���Լ�3������������ӷ�Ӧ����ʽ��_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��![]() ��һ����Ҫ�Ļ���ԭ�ϣ������ںϳɿɽ���ĸ߾���PES��֬�Լ����п��������ԵĻ�����K��
��һ����Ҫ�Ļ���ԭ�ϣ������ںϳɿɽ���ĸ߾���PES��֬�Լ����п��������ԵĻ�����K��

��֪:i.R1CO18OR2+R3OH![]() R1COOR3+R218OH
R1COOR3+R218OH
ii. 
��.  (R1��R2��R3��������)
(R1��R2��R3��������)
��1��A��������_________��C�Ĺ����ŵ�������_________��
��2��B����Ϊ��״�ṹ���˴Ź�������ֻ��һ��壬B�Ľṹ��ʽΪ_________ .
��3��E�����к���������������Ϊ˳ʽ�ṹ��E�Ľṹ��ʽΪ_________ .
��4����Ӧ���Ļ�ѧ����ʽΪ_________ ��
��5���Լ�a�Ľṹ��ʽΪ_________����Ӧ�������ķ�Ӧ����Ϊ________��Ӧ��
��6����֪:  ����1��3-����ϩΪ��ʼԭ�ϣ������֪��Ϣѡ�ñ�Ҫ�����Լ��ϳ�
����1��3-����ϩΪ��ʼԭ�ϣ������֪��Ϣѡ�ñ�Ҫ�����Լ��ϳ�![]() �������ºϳ�·�߲�������:_________________
�������ºϳ�·�߲�������:_________________

��7����֪����(-NH2)���ǻ����ƣ�Ҳ�ܷ�����Ӧi������J�Ʊ�K�Ĺ����У��������������L��L����ʽΪC16H13NO3����������Ԫ������L�Ľṹ��ʽΪ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������ˮ��Һ�������ֺ������壬25��ʱ�����ǵ����ʵ���������pH�ı仯��ͼ��ʾ�����������������

A. ��֪H3FeO4+�ĵ����ⳣ���ֱ�Ϊ��K1=2.5��10-2��K2=4.8��10-4��K3=5.0��10-8����pII=4������Һ��![]()
B. Ϊ��þ����ܴ����ĸ������Σ�Ӧ����pH��9
C. ��pH=5�ĸ���������Һ�м���KOH��Һ��������Ӧ�����ӷ���ʽΪHFeO4��+OH��=FeO42-+H2O
D. pH=2ʱ����Һ����Ҫ��������Ũ�ȵĴ�С��ϵΪc(H2FeO4)>c(H3FeO4+)> c (HFeO4��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ҵ�Ͽ�ͨ��ú��Һ���ϳɼ״�������ӦΪ:
CO(g)+2H2(g)![]() CH3OH(l) ��H=x��
CH3OH(l) ��H=x��
(1)��֪������CH3OH��H2��CO ��ȼ���ȷֱ�Ϊ726.5 kJ/mol��285.5 kJ/mol��283.0 k J/mol����x=____��Ϊ��ߺϳɼ״���Ӧ��ѡ���ԣ��ؼ�������__________________________��
(2)TK�������ݻ�Ϊ1.00 L��ij�ܱ������н���������Ӧ,���������ͼһ��
���û�ѧ��Ӧ0~10 min��ƽ������v(H2)=_______��M��N����淴Ӧ���ʽϴ����_____(����v��(M)������v��(N)����������ȷ����)��
��10 minʱ������CO���������Ϊ______��
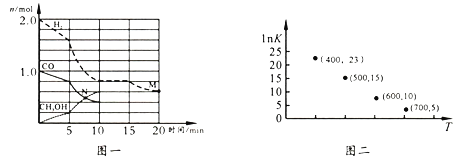
���������෴Ӧ������ij���(B)��ƽ��ѹǿ(pB)�������ʵ���Ũ��(cB)��ʾƽ�ⳣ��(��KP��ʾ)��������pB=p����B���������������TK ��ƽ��������ѹǿΪx atm����÷�ӦKP=____(�������ʽ)��ʵ���ò�ͬ�¶��µ�lnK(��ѧƽ�ⳣ��K ����Ȼ����)��ͼ���������1nK ��T���������仯���Ƶ�ԭ����________________��
(3)����ļ״�����������ȼ�ϵ�ء�
���о���Ա��������NaOH ����״�ʱ���¶ȿ��Ʋ������м����κ�H2�������䷴Ӧ����ʽΪ______________________________��
��ij��У�����CH3OH-O2ȼ�ϵ������Դ����ˮ�೧������CO2(������̼����Ϊ����)������ΪC ��O2���������缫��ӦʽΪ___________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����N2��H2�Ļ������ֱ����ס��ҡ������������У����кϳɰ���Ӧ��������ͬ��һ��ʱ���÷�Ӧ���ʷֱ�Ϊ���ף�v��H2����3 mol��L��1��min��1��
�ң�v��N2����2 mol��L��1��min��1������v��NH3���� 1 mol��L��1��min��1��
�����������кϳɰ��ķ�Ӧ���ʣ� ��
A. v(��)��v(��)��v(���� B. v(��)��v(��)��v(�ף�
C. v(��)��v(��)��v(��) D. v(��)��v(��)��v(����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����̼����(Na2CO4)��һ�ֺܺõĹ�����������ϡ���ᷢ����Ӧ�Ļ�ѧ����ʽΪ��2Na2CO4+4HCl=4NaCl+2CO2��+O2��+2H2O�����۹�̼����һ�㶼����̼���ƣ�Ϊ�ⶨij��̼������Ʒ(ֻ��Na2CO4��Na2CO3)�Ĵ��ȣ�ij��ѧ��ȤС������������ַ���ʵʩ��
����һ��

(1)�����ٺ͢۵����Ʒֱ�Ϊ_______��________��
(2)���������У�ʹ�õ�����������________(��������)��
(3)����������۵IJ�������___________��
������������ͼ��װ��ʵ��װ�ã�QΪһ�ɹ��͵�����������ȡ������Ʒ�����У���Һ©����������ϡ�����������������ַ�Ӧ

(4)Ϊ�ⶨ��Ӧ������������������ϡ����ǰ����ر�____��____ (���K1������K2����K3��)������A��������________��
(5)��������Ӧֹͣ��ʹK1��K3���ڹر�״̬��K2���ڴ�״̬���ٻ�����K1��B��װ�Ĺ����Լ���_________��ΪʲôҪ������K1?_______________��
(6)ʵ�����ʱ����Ͳ1����xmLˮ����ͲII���ռ�����ymL���壬����Ʒ�й�̼���Ƶ�����������________(�ú���x��y�Ĵ���ʽ��ʾ)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ӧ4NH3��g����5O2��g��![]() 4NO��g����6H2O��g����5L�ܱ������н��У�����Ӻ�NO�����ʵ���������0.3mol����˷�Ӧ��ƽ������vΪ
4NO��g����6H2O��g����5L�ܱ������н��У�����Ӻ�NO�����ʵ���������0.3mol����˷�Ӧ��ƽ������vΪ
A. v��O2����0.01mol/��L��s�� B. v��NO����0.08mol/��L��s��
C. v��H2O����0.003mol/��L��s�� D. v��NH3����0.001mol/��L��s��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ӦA��g��+3B��g��![]() 2C��g��+2D��g���������ֲ�ͬ������ò�ͬ���ʱ�ʾ�ķ�Ӧ���ʷֱ����£����з�Ӧ���������ǣ� ��
2C��g��+2D��g���������ֲ�ͬ������ò�ͬ���ʱ�ʾ�ķ�Ӧ���ʷֱ����£����з�Ӧ���������ǣ� ��
A. v��A��=0.15mol/��Lmin��
B. v��B��=0.04mol/��Ls��
C. v��C��=0.03mol/��Ls��
D. v��D��=0.4mol/��Lmin��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com