



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| ѡ�� | ʵ����� | ���� | ���ͻ���� |
| A | ������Fe���м���HNO3�� ��ַ�Ӧ����KSCN��Һ | ��Һ�ʺ�ɫ | ϡHNO3��Fe����Ϊ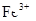 |
| B | AgI�����е���ϡKCl��Һ | �а�ɫ�������� | AgCl��AgI������ |
| C | Al������ϡHNO3�� | ������ | Al�����汻HNO3�������γ����ܵ�����Ĥ |
| D | �ò�����պȡŨ��ˮ�㵽��ɫʯ����ֽ�� | ��ֽ����ɫ | Ũ��ˮ�ʼ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
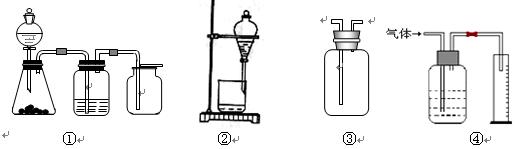
| A��װ�â���һ��ʵ��������װ�ã����ڷ�����������ռ����壬��ͭм��ϡ���� |
| B��װ�âڿ�����CCl4��ȡ��ˮ�еĵⲢ��Һ |
| C��װ�âۿ������ռ�H2��NH3��Cl2��HCl��NO2�� |
| D��װ�â������ڲ������������װ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���������ķ�������Fe��OH��3���� |
| B����ˮ���𱽡����Ȼ�̼���Ҵ�������ɫҺ�� |
| C�����ó���ʯ��ˮ����Na2CO3��Һ��NaHCO3��Һ |
| D��Ϊȷ�ⶨ������NaOH��Һ��Ӧ���к��ȣ�������ͼ�����ʵ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 ��֪��
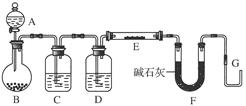
��֪��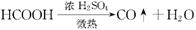 ��ʵ��������ͼ��ʾ��װ��.����ȡһ����̼��ѡ�õ�װ��Ϊ________________(����ĸ����
��ʵ��������ͼ��ʾ��װ��.����ȡһ����̼��ѡ�õ�װ��Ϊ________________(����ĸ����
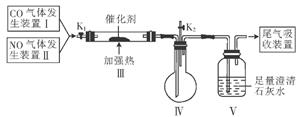
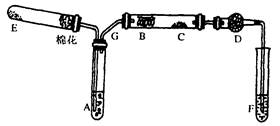
 ֹͣ���ȣ���K2��ͨ������������װ��IV�пɹ۲쵽�к���ɫ��������.��������________________(�ѧʽ����
ֹͣ���ȣ���K2��ͨ������������װ��IV�пɹ۲쵽�к���ɫ��������.��������________________(�ѧʽ�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����������ƽ�������壬���岻��ֱ�ӷ����������У���Ӧ�÷���ֽƬ�ϳ��� |
| B����Һʱ���²�Һ���ȴ��¿ڷų�����һ�������������ϲ�Һ��Ҳ���¿ڷų� |
| C��Ũ���С��մ��Ƥ���ϣ������ò��������ô���ˮ��ϴ�����Ϳ����С�մ���Һ |
| D����pH��ֽ��δ֪��Һ��pHʱ��������ʪ��ֽ�ٲ�������pHƫС |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
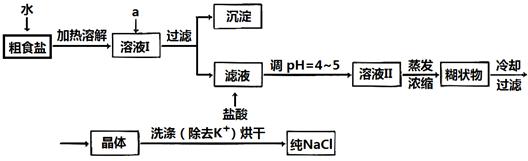
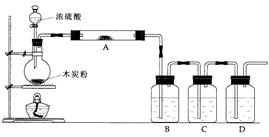
 ������ƽ��ҩ�ס����������___ _____�����������ƣ���
������ƽ��ҩ�ס����������___ _____�����������ƣ���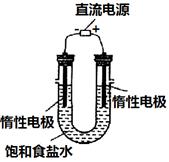
 MnCl2+Cl2��+2H2O���ݴˣ���������������װ����ѡ���Ʊ����ռ�H2��װ��_______(�����)���Ʊ����ռ��������Cl2��װ��_________������ţ���
MnCl2+Cl2��+2H2O���ݴˣ���������������װ����ѡ���Ʊ����ռ�H2��װ��_______(�����)���Ʊ����ռ��������Cl2��װ��_________������ţ���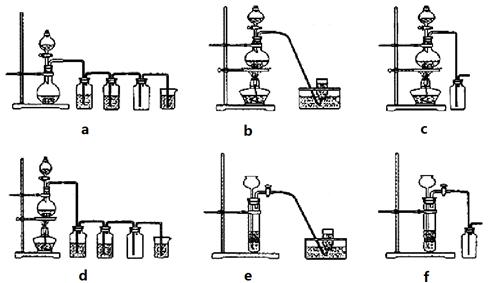
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com