
��11 �֣���ѧ��һֱ�����о����¡���ѹ�¡��˹��̵������·���������ʵ�鱨�����ڳ��¡���ѹ�����������£�N2�ڴ�������������Fe2O3��TiO2��������ˮ������Ӧ�����ɵ���Ҫ����ΪNH3����Ӧ�Ļ�ѧ����ʽ���£�N2(g)+ 3H2O(l)  2NH3(g)+ O2(g)���ش��������⣺
2NH3(g)+ O2(g)���ش��������⣺
��1����һ���о�NH3���������¶ȵĹ�ϵ������ʵ�����ݼ��±������ա�N2ѹ��1.0��105 Pa����Ӧʱ��3 h������÷�Ӧ������ӦΪ ��Ӧ������ȡ����ȡ���
|
T/K |
303 |
313 |
323 |
|
NH3������/��10-6 mol�� |
4.8 |
5.9 |
6.0 |
��2����Ŀǰ�㷺ʹ�õĹ�ҵ�ϳɰ�������ȣ��÷����й̵���Ӧ�������������������䷴Ӧ����������NH3�������Ľ��飺���� ��
��3���ϳɰ���ҵ��ԭ����N2�ɴӿ����з���õ���H2���ü����ڸ�������ˮ������Ӧ�Ƶá������ڸ�������ˮ������Ӧ��Ӧ����ʽΪ��CH4(g)��H2O(g)��CO(g)��3H2(g)���������ʵ�ȼ������
�����£�
H2(g) ����H =��285.8 kJ・mol��1��
CO(g) �� ��H =��283.0 kJ・mol��1��
CH4(g) ����H =��890.3 kJ・mol��1 ��
��֪1mol H2O(g)ת��Ϊ1mol H2O(l)ʱ�ų�44.0 kJ������д��CH4��H2O�ڸ����·�Ӧ���Ȼ�ѧ����ʽ__________________________________��
��4����������Ѱ���ʺϵĴ����͵缫���ϣ���N2��H2Ϊ�缫��Ӧ���HCl����NH4ClΪ�������Һ�Ƴ�����ȼ�ϵ�أ���д���õ缫��������Ӧʽ
��5�����ɵ�NH3���������̬���ʣ���(NH4)2SO4��NH4Cl����Щ������ �ԣ�ԭ���ǣ������ӷ���ʽ��ʾ��___________________________��ʹ��ʱ������________________���ʺ�ʩ��
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭�ٺ��а��Ƹ���ѧ��һ��ѧ�����п��Ի�ѧ������������ ���ͣ������
��ÿ��1�֣���6�֣���ѧ������̽���������صĹ����У���ʶ������ϸ������ɺ�Ԫ���������������е���ϵ��Լռ����������99.97%��11�ִ���Ԫ��ȫ����Ԫ�����ڱ�ǰ20��Ԫ�أ�����0.03%����10�������岻��ȱ�ٵ���Ԫ����ɡ�����a��h 8�ֶ�����Ԫ�أ��dz������ء��������������Ԫ�أ�������Ԫ�����ڱ��е�λ�����£���ݴ˻ش��������⣺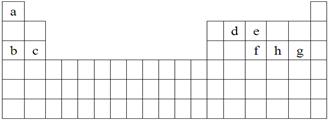
��1�������е�Ԫ�ص�ԭ�Ӽ䷴Ӧ�������γ����Ӽ����� ��
A��c��f B��b��g C��d��g D��b��e
��2��������a��g�γɵĸ�����������ԭ�Ӷ����������Ϊ8���ӽṹ���� ��
A��ea3 B��ag C��fg3 D��dg4
��3����a��e��ɵ�һ�ֻ����ﻯѧʽΪea5�����ԭ�Ӿ��ﵽͬ����ϡ������ԭ�ӵ��ȶ��ṹ����д���û�����ĵ���ʽ ������������ ������ӡ����ۡ��������
��4������a��b��h��������Ԫ���γɵ��������ӻ�������Ӧ�������д̼�����ζ�����塣�����ֻ���������һ����Է�������Ϊ120���û������ۻ�ʱ�ƻ����� ���ѧ�����ͣ���ͬ��������ˮʱ�ƻ����� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���˽̰���л�ѧ���� 1.1Ԫ�����ڱ���ϰ���������棩 ���ͣ������
(11��)��W��X��Y��Z����Ԫ�أ�����WԪ����1826��һλ���������ѧ�ҷ��ֵġ������о���ˮ����ʱ����ʣ��ĸ������±��ͨ������������Һ��ɫ����پ�����һ����ȡ�ɵ�һ�ֺ���ɫ�д̱dz�ζ��Һ�壬�����WԪ�صĵ��ʡ�X��Y��Z����������Ԫ�أ�ZԪ����������Ǹ��۾���ֵ��3��������������Yԭ�Ӻ�����������2����Xԭ�Ӻ���ĵ�������Z ԭ�ӵ����ڲ��ϵ�������һ�롣
��W��X��Y��Z��Ԫ�ط��ŷֱ��ǣ�W X Y Z
��W��ԭ�ӽṹʾ��ͼΪ ��Wλ�ڵ� ���� �塣
�ǿ�±��ͨ������������Ӧ�����ӷ���ʽΪ ��
����WԪ�صĵ�����ZY2��X2Y��Ӧ��������������Ļ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014���㽭�ٺ��и�һ��ѧ�����п��Ի�ѧ���������棩 ���ͣ������
��ÿ��1�֣���6�֣���ѧ������̽���������صĹ����У���ʶ������ϸ������ɺ�Ԫ���������������е���ϵ��Լռ����������99.97%��11�ִ���Ԫ��ȫ����Ԫ�����ڱ�ǰ20��Ԫ�أ�����0.03%����10�������岻��ȱ�ٵ���Ԫ����ɡ�����a��h 8�ֶ�����Ԫ�أ��dz������ء��������������Ԫ�أ�������Ԫ�����ڱ��е�λ�����£���ݴ˻ش��������⣺

��1�������е�Ԫ�ص�ԭ�Ӽ䷴Ӧ�������γ����Ӽ����� ��
A��c��f B��b��g C��d��g D��b��e
��2��������a��g�γɵĸ�����������ԭ�Ӷ����������Ϊ8���ӽṹ���� ��
A��ea3 B��ag C��fg3 D��dg4
��3����a��e��ɵ�һ�ֻ����ﻯѧʽΪea5�����ԭ�Ӿ��ﵽͬ����ϡ������ԭ�ӵ��ȶ��ṹ����д���û�����ĵ���ʽ ������������ ������ӡ����ۡ��������
��4������a��b��h��������Ԫ���γɵ��������ӻ�������Ӧ�������д̼�����ζ�����塣�����ֻ���������һ����Է�������Ϊ120���û������ۻ�ʱ�ƻ����� ���ѧ�����ͣ���ͬ��������ˮʱ�ƻ����� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�긣��ʡ�����и�����ѧ��12���¿���ѧ�Ծ� ���ͣ������
��ѧ��Ԥ�ԣ�ȼ�ϵ�ؽ���21���ͻ�õ�������Ҫ;���������Ѽƻ����״�ȼ�ϵ�����ھ���Ŀ�ġ�һ�ּ״�(CH3OH)ȼ�ϵ���Dz��ò���̼������Ϊ�缫��������ϡ������Һ��ֱ�Ӽ��봿����ļ״���ͬʱ��һ���缫ͨ��������ش��������⣺(11��)
��1�����ֵ缫�ŵ�ʱ�������ܷ�Ӧ��______________________________________��
��2���˵缫�����������ĵ缫��Ӧʽ��__________________________��
���������ĵ缫��Ӧʽ��_______________________________��
��3�����Һ�е�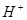 ������_________�������������ƶ���ͨ��������������________.
������_________�������������ƶ���ͨ��������������________.
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��11��)2005��1��������ѧ����Science�Ϸ������ģ�����������Al�ij�ԭ�ӽṹ����Ԥ����������ԭ��Ҳ���ܴ������ƵĽṹ������һ��Ի�ѧ�������Լ�������������ش�Ӱ��ķ��֣������˿�ѧ��Ĺ㷺��ע�����ֳ�ԭ������Al�ĵ⻯���з��ֵģ���13��Alԭ�ӻ�14��Alԭ���γ�Al13��Al14��ԭ�ӽṹ�����ӻ�ѧ������������Al13�γ�12��Al�ڱ��棬1��Al�����ĵ����Ƕ�ʮ����ṹ��Al14���Կ�����һ��Alԭ�Ӹ�Al13���ϵ�һ�������ε�3��Al�γ�Al�DAl������õġ����»�ָ����All3��All4��ԭ�Ӷ��Ǿ���40���۵���ʱ���ȶ���
(1) ����������Ϣ��Ԥ��Al13��Al14���ȶ����ϼ�̬�ֱ�Ϊ �� ��A114Ӧ����Ԫ�����ڱ��� �ѧԪ�ص����ʣ������ǣ� ��
(2) ��Al13��A114��Al�DAl�����IJⶨʮ�����ѣ������ۼ��������Al13����Al14�е�Al�DAl�������������Al�DAl�����൱����֪�������ľ���ṹ��ȡ�����������ܶѻ����ܶ�ԼΪ2.7g/cm3�������Al13��Al14����Al�DAl�ļ�����
��
(3) Al13���Ƕ�ʮ�������ж��ٸ��������϶������Ϊ���������϶����������в�������ԭ�ӣ���ͨ��������ƿɲ���ԭ�ӵİ뾶���Ϊ����?
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com