
��14�֣��������ķ������ᴿ�ڻ�ѧʵ����ռ����Ҫ��λ�á���ͼ��ʾ�ӹ��������з���X�ķ�������ش��й����⡣
��1��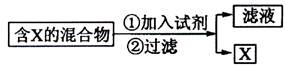
����������ͼʾ��ij������ĩ(����Au��Ag��Cu)�з���Au��������Լ��� ���йط�Ӧ�Ļ�ѧ����ʽΪ ��
��2��Ϊ�ᴿijFe2O3��Ʒ(��Ҫ������SiO2��A12O3)�������һ���Կ�ͼ��ʽ��ʾ��ʵ�鷽��(ע�����ʺͲ���) ��
����ҵ���Ի�ͭ��Ϊԭ�ϣ����û�������������ͭ��
��1���ù��յ��м���̻ᷢ����Ӧ�� ����Ӧ����������______________����������Ϊ__________
����Ӧ����������______________����������Ϊ__________
��2���ھ���ͭ�Ĺ����У����Һ��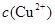 ���½���
���½���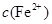 ��
��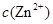 �������ӣ������趨ʱ��ȥ���е�
�������ӣ������趨ʱ��ȥ���е�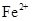 ��
��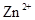 ���±�Ϊ�������ʵ��ܶȻ���
���±�Ϊ�������ʵ��ܶȻ���
| ���� |  | 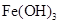 | 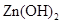 |  |
�ܶȻ�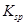 | 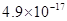 | 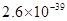 | 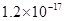 | 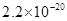 |
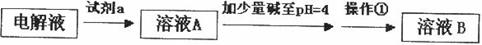
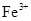 ����
���� ����������ȥ������ͬѧ��Ϊ��ͬѧ�ķ�����Ҳ
����������ȥ������ͬѧ��Ϊ��ͬѧ�ķ�����Ҳ �п������п��Ծ����ϵ�д�
�п������п��Ծ����ϵ�д� ��������״Ԫ��ϵ�д�
��������״Ԫ��ϵ�д� �ƸԿ�����ҵ��ϵ�д�
�ƸԿ�����ҵ��ϵ�д� ��Ԫ����ĩ��ϰ�ȷ��ϵ�д�
��Ԫ����ĩ��ϰ�ȷ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| ʵ�鲽�� | �й����� |
| �ټ�������NaCl������ | |
| �ڳ���NaCl���� | ��������ƽ��Ҫ����NaCl������Ϊ 2.9 2.9 g�� |
| �۽�NaCl����100mL�ձ��У�����������ˮ | Ϊ�˼ӿ��ܽ����ʣ����Բ�ȡ��Щ��ʩ�� �ò��������� �ò��������� �� |
| �ܽ��ձ�����Һת����250mL����ƿ�� | Ϊ�˷�ֹ��Һ������Ӧ��ȡʲô��ʩ�� �ò��������� �ò��������� �� |
| ��������ƿ�м�����ˮ���̶��� | �ڽ��д˲���ʱ����ˮ���̶���1-2���״�Ӧ��β����� ���ý�ͷ�ιܼ�ˮ���̶��� ���ý�ͷ�ιܼ�ˮ���̶��� �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ķ������ᴿ�ڻ�ѧʵ����ռ����Ҫ��λ�á���ͼ��ʾ�ӹ��������з���X�ķ�������ش��й����⡣
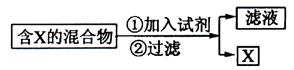
![]() ��1��
��1��
����������ͼʾ��ij������ĩ(����Au��Ag��Cu)�з���Au��������Լ��� ���йط�Ӧ�Ļ�ѧ����ʽΪ ��
![]() ��2��Ϊ�ᴿijFe2O3��Ʒ(��Ҫ������SiO2��A12O3)�������һ���Կ�ͼ��ʽ��ʾ��ʵ�鷽��(ע�����ʺͲ���) ��
��2��Ϊ�ᴿijFe2O3��Ʒ(��Ҫ������SiO2��A12O3)�������һ���Կ�ͼ��ʽ��ʾ��ʵ�鷽��(ע�����ʺͲ���) ��
����ҵ���Ի�ͭ��Ϊԭ�ϣ����û�������������ͭ��
��1���ù��յ��м���̻ᷢ����Ӧ�� ����Ӧ����������______________����������Ϊ__________
����Ӧ����������______________����������Ϊ__________
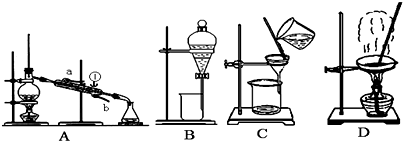
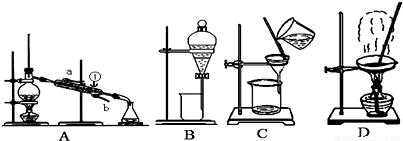
��2���ھ���ͭ�Ĺ����У����Һ��![]() ���½���
���½���![]() ��
��![]() �������ӣ������趨ʱ��ȥ���е�
�������ӣ������趨ʱ��ȥ���е�![]() ��
��![]() ���±�Ϊ�������ʵ��ܶȻ���
���±�Ϊ�������ʵ��ܶȻ���
| ���� |
|
|
|
|
| �ܶȻ� |
|
|
|
|
��ͬѧ��������³��ӷ�����
![]()
���Լ�a��__________����Ŀ����____________________________________�������ܶȻ��÷����ܹ���ȥ�����ʽ�����������____________��д����������ʽ��������ӵIJ���������____________________________________________________________________________.
����ͬѧ�ڲ�������ʱ���֣�����ҵԭ���Ȼ���к������Ȼ�����ʹ������ˮ���ټ��백ˮ
����pH��7��8����ʹ![]() ����
����![]() ����������ȥ������ͬѧ��Ϊ��ͬѧ�ķ�����Ҳ
����������ȥ������ͬѧ��Ϊ��ͬѧ�ķ�����Ҳ
Ӧ�ý���ҺpH����7��8������Ϊ��ͬѧ�Ľ����Ƿ���ȷ?________(��ǡ���)��
������________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| ʵ�鲽�� | �й����� |
| �ټ�������NaCl������ | |
| �ڳ���NaCl���� | ��������ƽ��Ҫ����NaCl������Ϊ______g�� |
| �۽�NaCl����100mL�ձ��У�����������ˮ | Ϊ�˼ӿ��ܽ����ʣ����Բ�ȡ��Щ��ʩ��______�� |
| �ܽ��ձ�����Һת����250mL����ƿ�� | Ϊ�˷�ֹ��Һ������Ӧ��ȡʲô��ʩ��______�� |
| ��������ƿ�м�����ˮ���̶��� | �ڽ��д˲���ʱ����ˮ���̶���1-2���״�Ӧ��β����� ______�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ���Ĵ�ʡ�ɶ���˫�����ĺ���ѧ��һ���ϣ����л�ѧ�Ծ��������棩 ���ͣ������
| ʵ�鲽�� | �й����� |
| �ټ�������NaCl������ | |
| �ڳ���NaCl���� | ��������ƽ��Ҫ����NaCl������Ϊ______g�� |
| �۽�NaCl����100mL�ձ��У�����������ˮ | Ϊ�˼ӿ��ܽ����ʣ����Բ�ȡ��Щ��ʩ��______�� |
| �ܽ��ձ�����Һת����250mL����ƿ�� | Ϊ�˷�ֹ��Һ������Ӧ��ȡʲô��ʩ��______�� |
| ��������ƿ�м�����ˮ���̶��� | �ڽ��д˲���ʱ����ˮ���̶���1-2���״�Ӧ��β����� ______�� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com