
 A��B��C��D��E��F����ǰ��������ԭ�������������������Ԫ�أ�����A��B��C��ͬһ�������ڵ�����Ԫ�أ�B���⻯��������C���⻯���У�D�Ļ�̬ԭ��s�����p����ϵĵ�����֮��Ϊ2��3����DΪ����Ԫ�أ�E��FΪ����Ԫ�أ�4s�ܼ���ֻ��һ�����ӣ��ش��������⣺
A��B��C��D��E��F����ǰ��������ԭ�������������������Ԫ�أ�����A��B��C��ͬһ�������ڵ�����Ԫ�أ�B���⻯��������C���⻯���У�D�Ļ�̬ԭ��s�����p����ϵĵ�����֮��Ϊ2��3����DΪ����Ԫ�أ�E��FΪ����Ԫ�أ�4s�ܼ���ֻ��һ�����ӣ��ش��������⣺���� A��B��C��D��E��F����ǰ��������ԭ�������������������Ԫ�أ�����A��B��C��ͬһ�������ڵ�����Ԫ�أ�B���⻯��������C���⻯���У�BΪNԪ�أ�CΪOԪ�أ�AӦΪCԪ�أ�D�Ļ�̬ԭ��s�����p����ϵĵ�����֮��Ϊ2��3��ӦΪPԪ�أ���������Ų�Ϊ1s22s22p63s22p3��E��FΪ����Ԫ�أ�4s�ܼ���ֻ��һ�����ӣ���������Ų�ʽ�ֱ�Ϊ��1s22s22p63s22p63d54s1��1s22s22p63s22p63d104s1����EΪCrԪ�ء�FΪCuԪ�أ��ݴ˽��н��1������5����
��6���ٸ����£�B�����廯���P�����廯���������ķ�Χ�кϳ�BP�����ݷ�Ӧ������P��Ӧ������д����ʽ��
�ھ�����B��Pԭ�ӵ��������Ϊanm����a��10-7cm�� ��ͼ��1��2��3��4ԭ���γ���������ṹ�����ĸ�ԭ��֮���Bԭ��λ���������������ϣ�1��5ԭ��֮��ľ���Ϊa��10-7cm�����㵽���ľ��������ĵ��������֮��Ϊ3��1�������Ϊ$\frac{4}{3}$��a��10-7cm����1��2ԭ��֮�����Ϊx��1��2��3ԭ���γɵ��������ε���ĸ�=$\frac{\sqrt{3}}{2}$x���������ζ��㵽���ĵľ��������ĵ��ľ���֮��Ϊ2��1�������������ζ��㵽���ĵľ���=$\frac{\sqrt{3}}{2}$x��$\frac{2}{3}$=$\frac{\sqrt{3}}{3}$x����1��2��5����ֱ���������д���x2=��$\frac{\sqrt{3}}{3}$x��2+��$\frac{4}{3}$��a��10-7��2��x=$\frac{2\sqrt{6}}{3}$��a��10-7�����ⳤ=$\sqrt{\frac{��2x��^{2}}{2}}$cm=$\frac{4}{\sqrt{3}}a��1{0}^{-7}$cm���������=��$\frac{4}{\sqrt{3}}a��1{0}^{-7}$��3cm3��
��ͼ��1��2��3��4ԭ���γ���������ṹ�����ĸ�ԭ��֮���Bԭ��λ���������������ϣ�1��5ԭ��֮��ľ���Ϊa��10-7cm�����㵽���ľ��������ĵ��������֮��Ϊ3��1�������Ϊ$\frac{4}{3}$��a��10-7cm����1��2ԭ��֮�����Ϊx��1��2��3ԭ���γɵ��������ε���ĸ�=$\frac{\sqrt{3}}{2}$x���������ζ��㵽���ĵľ��������ĵ��ľ���֮��Ϊ2��1�������������ζ��㵽���ĵľ���=$\frac{\sqrt{3}}{2}$x��$\frac{2}{3}$=$\frac{\sqrt{3}}{3}$x����1��2��5����ֱ���������д���x2=��$\frac{\sqrt{3}}{3}$x��2+��$\frac{4}{3}$��a��10-7��2��x=$\frac{2\sqrt{6}}{3}$��a��10-7�����ⳤ=$\sqrt{\frac{��2x��^{2}}{2}}$cm=$\frac{4}{\sqrt{3}}a��1{0}^{-7}$cm���������=��$\frac{4}{\sqrt{3}}a��1{0}^{-7}$��3cm3��
�þ�����Bԭ�Ӹ���Ϊ4��Pԭ�Ӹ���=8��$\frac{1}{8}$+6��$\frac{1}{2}$=4���ٸ����ܶ�=$\frac{m}{V}$���㣮
��� �⣺A��B��C��D��E��F����ǰ��������ԭ�������������������Ԫ�أ�����A��B��C��ͬһ�������ڵ�����Ԫ�أ�B���⻯��������C���⻯���У�BΪNԪ�أ�CΪOԪ�أ�AӦΪCԪ�أ�D�Ļ�̬ԭ��s�����p����ϵĵ�����֮��Ϊ2��3��ӦΪPԪ�أ���������Ų�Ϊ1s22s22p63s22p3��E��FΪ����Ԫ�أ�4s�ܼ���ֻ��һ�����ӣ���������Ų�ʽ�ֱ�Ϊ��1s22s22p63s22p63d54s1��1s22s22p63s22p63d104s1����EΪCrԪ�ء�FΪCuԪ�أ�
��1��ͬһ�����У�ԭ������Խ�縺��ԽС��������Ԫ�ذ��縺����С�����˳��Ϊ��C O N��C��O��N��
�ʴ�Ϊ��C O N��C��O��N��
��2��BΪNԪ�أ�����Ԫ���γɵ���ԭ��������ΪNO3-��NO3-������Cԭ�Ӽ۲���Ӷ���=3+$\frac{5+1-2��3}{2}$=3����Nԭ�Ӳ����µ��Ӷԣ�������ռ乹��Ϊƽ�������Σ�ԭ�Ӳ���sp2�ӻ��������Ȼ���ΪNCl3��������к���3��������1���¶Ե��ӣ��������Nԭ�Ӳ���sp3�ӻ������ӵ����幹��Ϊ�����Σ�
�ʴ�Ϊ��sp2�������Σ�
��3��ԭ�Ӹ�����ȡ��۵�������ȵ�����Ϊ�ȵ����壬��CN-���ӻ�Ϊ�ȵ�������У�C22-��O22+��
�ʴ�Ϊ��C22-��O22+��
��4��F�������ԭ�ӹ�����ڰ����״̬�������ܲ���������ӣ�ΪCu��ԭ������Ϊ29��ԭ�Ӻ�������Ų�Ϊ��1s22s22p63s23p63d104s1����Χ�����Ų�ʽΪ��3d104s1��Cu�������������������ܶѻ���ΪABC�����з�ʽ���ʢܷ��ϣ�
�ʴ�Ϊ��3d104s1���ܣ�
��5����1.0mol�þ������Һ�м�������AgNO3��Һ����143.5g��ɫ�������ð�ɫ����ΪAgCl�������ʵ���Ϊ��$\frac{143.5g}{143.5g/mol}$=1mol��˵���ڸû������к���1�������ӣ�Cr3+��Cl-��H2O�����ʵ���֮��Ϊ1��3��6������λ�����к�����������Ϊ��3-1=2����λ�����к���ˮΪ��6-2=4�����нᾧˮˮ�ĸ���Ϊ��6-4=2���ʸ���λ������Ļ�ѧʽΪ��[Cr��H2O��4Cl2]Cl•2H2O��
�ʴ�Ϊ��[Cr��H2O��4Cl2]Cl•2H2O��
��6���ٸ����£�B�����廯���P�����廯���������ķ�Χ�кϳ�BP�����ݷ�Ӧ������P��Ӧ������д����ʽΪBBr3+PBr3+3H2$\frac{\underline{\;����\;}}{\;}$BP+6HBr��
�ʴ�Ϊ��BBr3+PBr3+3H2 $\frac{\underline{\;����\;}}{\;}$ BP+6HBr��
�ھ�����B��Pԭ�ӵ��������Ϊapm����a��10-7cm�� ��ͼ��1��2��3��4ԭ���γ���������ṹ�����ĸ�ԭ��֮���Bԭ��λ���������������ϣ�1��5ԭ��֮��ľ���Ϊa��10-7cm�����㵽���ľ��������ĵ��������֮��Ϊ3��1�������Ϊ$\frac{4}{3}$��a��10-7cm����1��2ԭ��֮�����Ϊx��1��2��3ԭ���γɵ��������ε���ĸ�=$\frac{\sqrt{3}}{2}$x���������ζ��㵽���ĵľ��������ĵ��ľ���֮��Ϊ2��1�������������ζ��㵽���ĵľ���=$\frac{\sqrt{3}}{2}$x��$\frac{2}{3}$=$\frac{\sqrt{3}}{3}$x����1��2��5����ֱ���������д��ڣ�x2=��$\frac{\sqrt{3}}{3}$x��2+��$\frac{4}{3}$��a��10-7��2����ã�x=$\frac{2\sqrt{6}}{3}$��a��10-7�����ⳤ=$\sqrt{\frac{��2x��^{2}}{2}}$cm=$\frac{4}{\sqrt{3}}a��1{0}^{-7}$cm���������=��$\frac{4}{\sqrt{3}}a��1{0}^{-7}$��3cm3��
��ͼ��1��2��3��4ԭ���γ���������ṹ�����ĸ�ԭ��֮���Bԭ��λ���������������ϣ�1��5ԭ��֮��ľ���Ϊa��10-7cm�����㵽���ľ��������ĵ��������֮��Ϊ3��1�������Ϊ$\frac{4}{3}$��a��10-7cm����1��2ԭ��֮�����Ϊx��1��2��3ԭ���γɵ��������ε���ĸ�=$\frac{\sqrt{3}}{2}$x���������ζ��㵽���ĵľ��������ĵ��ľ���֮��Ϊ2��1�������������ζ��㵽���ĵľ���=$\frac{\sqrt{3}}{2}$x��$\frac{2}{3}$=$\frac{\sqrt{3}}{3}$x����1��2��5����ֱ���������д��ڣ�x2=��$\frac{\sqrt{3}}{3}$x��2+��$\frac{4}{3}$��a��10-7��2����ã�x=$\frac{2\sqrt{6}}{3}$��a��10-7�����ⳤ=$\sqrt{\frac{��2x��^{2}}{2}}$cm=$\frac{4}{\sqrt{3}}a��1{0}^{-7}$cm���������=��$\frac{4}{\sqrt{3}}a��1{0}^{-7}$��3cm3��
�þ�����Bԭ�Ӹ���Ϊ4��Pԭ�Ӹ���Ϊ��8��$\frac{1}{8}$+6��$\frac{1}{2}$=4��
���Ըþ����ܶ�Ϊ��$\frac{m}{V}$=$\frac{\frac{M}{{N}_{A}}��4}{V}$=$\frac{\frac{42}{{N}_{A}}��4}{��\frac{4}{\sqrt{3}}{��a��1{0}^{-7}��}^{3}}$g/cm3=$\frac{4��42}{{N}_{A}����\frac{4}{\sqrt{3}}a��1{0}^{-10}��^{3}}$g/cm3��
�ʴ�Ϊ��$\frac{4��42}{{N}_{A}����\frac{4}{\sqrt{3}}a��1{0}^{-10}��^{3}}$��
���� ���⿼����λ�ýṹ�����ʹ�ϵ���ۺ�Ӧ�á������ļ��㣬��Ŀ�ѶȽϴ�ע����������ԭ�ӽṹ��Ԫ�����ڱ���Ԫ�������ɵĹ�ϵ���ƶ�Ԫ��Ϊ���ؼ�����6���о�������Ϊ�ѵ㡢�״��㣬ע�����վ�������ķ����뼼�ɣ�����������ѧ���ķ�����������������ѧ����������


 �����ܾ�ϵ�д�
�����ܾ�ϵ�д� ���ƿ�����ϵ�д�
���ƿ�����ϵ�д� ���¿쳵����������ϵ�д�
���¿쳵����������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
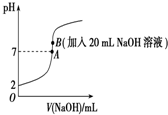 25���£���20mL 0.2mol•L-1��������еμ�0.2mol•L-1��NaOH��Һʱ����Һ��pH�仯��ͼ��ʾ����ش��������⣺
25���£���20mL 0.2mol•L-1��������еμ�0.2mol•L-1��NaOH��Һʱ����Һ��pH�仯��ͼ��ʾ����ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NH4Cl | B�� | Mg��HCO3��2 | C�� | H2SO4 | D�� | MgCl2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
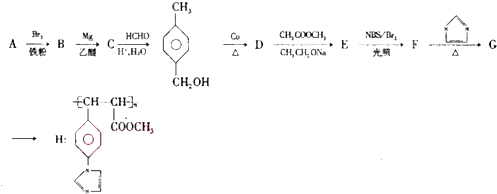



 ��
�� ��
�� ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

 ���÷�Ӧ������ȥ��Ӧ��
���÷�Ӧ������ȥ��Ӧ�� ��
�� ��
�� ��2��-CH3��
��2��-CH3�� �ı仯����
�ı仯���� ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 0.1mol/L CH3COOH��Һ��0.1mol/L NaOH��Һ�������ϣ�������Һ�У�c��OH-����c��H+��+c��CH3COOH�� | |
| B�� | �����£�pH=2��������pH=12�İ�ˮ�������ϣ�������Һ�У�c��Cl-����c��H+����c��NH4+ ����c��OH-�� | |
| C�� | 20ml 0.1mol/L CH3COONa��Һ��10ml HCl��Һ��Ϻ���Һ�����ԣ�������Һ�У�c��CH3COO-����c��Cl-����c��CH3COOH����c��H+����c��OH-�� | |
| D�� | 0.1mol/L NaHCO3��Һ��0.1mol/L NaOH��Һ�������ϣ�������Һ�У�c��Na+����c��CO32-����c��HCO3- ����c��OH-�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 ����̼���������������ַ��ӷ���������Ӧ��ȡ�������ַ��ӵ�ԭ�Ӹ�����Ϊ��������
����̼���������������ַ��ӷ���������Ӧ��ȡ�������ַ��ӵ�ԭ�Ӹ�����Ϊ��������| A�� | 3��5 | B�� | 1��2 | C�� | 2��3 | D�� | 2��5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������


 ��
��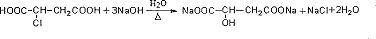 ��
�� �ȣ�����дһ�֣�
�ȣ�����дһ�֣��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com