
����Ŀ�����������(Na2S2O3)���������մ�����������ɫ���ĵ�б���壬�۵�48�档���������(Na2S2O3)����Ϊ����ҵ�Ķ�Ӱ������Ӧԭ��ΪAgBr+2Na2S2O3=Na3[Ag(S2O3)2]+NaBr��
��Ϊ�˴ӷ϶�ӰҺ����ȡ AgNO3���������ʵ�����̡�
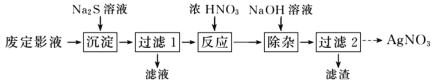
(1)������������������ Ag2S ���������������ȫ�IJ�����________��
(2)����Ӧ�������л����ɵ���ɫ���壬�÷�Ӧ�Ļ�ѧ����ʽΪ________��
(3)������ 2������Һ��ȡ AgNO3����IJ���������Ũ������ȴ�ᾧ��________��________�����
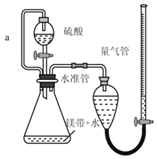
����ͼ��ʵ����ģ�ҵ�Ʊ� Na2S2O3 ��װ��ͼ��
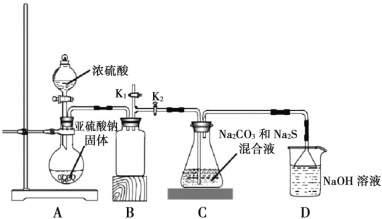
����ͼʾ�ش��������⣺
(4)װ�� A ��ʢ���������ƹ���IJ�������������________��װ�� B ��������________��
(5)��Һ©������ֱ���� 98����Ũ���ᣬ��ƿ�й����ײ�������顱����ʹ��Ӧ���ʱ�������������顱�����ԭ����________��
(6)���� K1���ܵ�Ŀ����Ϊ�˷�ֹ���װ��ʱ��ɿ�����Ⱦ���������������________��
(7)��������ƻ������ڳ�ȥ����Ƥ��ʱ�������ظ����Σ����仹ԭ�� Cr3+�������ϴ���1mol Cr2O72-��Ҫ Na2S2O3������Ϊ________��
���𰸡����ã�ȡ�ϲ���Һ�μ�Na2S��Һ���������г������ɣ���˵�������Ѿ���ȫ Ag2S+4HNO3=2AgNO3+2NO2��+S+2H2O ���� ϴ�� Բ����ƿ ��ֹ����(��ȫƿ) ���ɵ�Na2SO4���帽����Na2SO3���� ���齺�ܽ�K1�϶�����ͨ��װ��NaOH��Һ���ձ��У�Ȼ��ر�K2��K1 118.5g
��������
�ӷ϶�ӰҺ����ȡAgNO3���ڷ϶�ӰҺ{��Ҫ�ɷ�ΪNa3[Ag(S2O3)2]}�м���Na2S��Һ����Ag2S���������˺���Ũ�����ܽ�Ag2S����������������Һ�͵���ɫ���壬�õ���ɫ����Ϊ��������NaOH��ȥ���ʣ����˺�õ���������Һ��Ȼ����������Һ����Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����õ����������壻
��Aװ����Ũ�������������ƹ��巴Ӧ�ų������������壬���ڶ�������������ˮ��Bװ��Ϊ��ȫƿ��Cװ���ж�����������ͨ��̼���ƺ����ƵĻ����Һ�з�Ӧ����Na2S2O3��Һ��δ��Ӧ��ȫ�Ķ���������D�б�����������Һ���գ���ֹ��Ⱦ���ݴ˷������
��(1)������������������Ag2S���������������ȫ�IJ���Ϊ�����ã�ȡ�ϲ���Һ�μ�Na2S��Һ���������г������ɣ���˵�������Ѿ���ȫ���ʴ�Ϊ�����ã�ȡ�ϲ���Һ�μ�Na2S��Һ���������г������ɣ���˵�������Ѿ���ȫ��
(2) ��Ũ�����ܽ�Ag2S����������������Һ����ͬʱ�ų������������壬��Ӧ�Ļ�ѧ����ʽΪAg2S+4HNO3=2AgNO3+2NO2��+S+2H2O���ʴ�Ϊ��Ag2S+4HNO3=2AgNO3+2NO2��+S+2H2O��
(3)������ 2������Һ��ȡAgNO3����IJ���������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����ʴ�Ϊ�����ˣ�ϴ�ӣ�
��(4)����ͼʾ��װ�� A ��ʢ���������ƹ���IJ�������ΪԲ����ƿ����������������ˮ��װ�� B Ϊ��ȫƿ����ֹ�������ʴ�Ϊ��Բ����ƿ����ֹ����(��ȫƿ)��
(5)��Һ©������ֱ���� 98����Ũ���ᣬ��ƿ�й����ײ�������顱�������ɵ�Na2SO4���帽����Na2SO3���棬ʹ��Ӧ���ʱ������ʴ�Ϊ�����ɵ�Na2SO4���帽����Na2SO3���棻
(6)����K1���ܵ�Ŀ����Ϊ�˷�ֹ���װ��ʱ��ɿ�����Ⱦ�������������Ϊ�����齺�ܽ�K1�϶�����ͨ��װ��NaOH��Һ���ձ��У�Ȼ��ر�K2��K1���ʴ�Ϊ�����齺�ܽ�K1�϶�����ͨ��װ��NaOH��Һ���ձ��У�Ȼ��ر�K2��K1��
(7)��������ƻ������ڳ�ȥ����Ƥ��ʱ�������ظ����Σ����仹ԭ��Cr3+����Ӧ�ķ���ʽΪ3S2O32-+ 4Cr2O72-+ 26H+= 6SO42-+8Cr3++ 13H2O�����ݷ���ʽ�������ϴ���1mol Cr2O72-��Ҫ0.75mol Na2S2O3������Ϊ0.75mol��158g/mol=118.5g���ʴ�Ϊ��118.5g��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������һ�ֺ��м�ֵ�Ļ���ԭ�ϣ�������ɴX����֡����ӿɺϳ������Ľ�����ʹҩ������˾ƥ�֣�Ҳ�ɺϳɾ�̼��������ϳ�·�����£�

(1)��˾ƥ�ַ����еĺ�������������Ϊ_______��G�Ļ�ѧ������____��
(2)EΪ����һԪͪ����ṹ��ʽΪ________��H��K�ϳɾ�̼�����ķ�Ӧ������_____��
(3)����G�Ͱ�˾ƥ�ֵ�һ����ɫ�Լ�Ϊ___________
(4)��֪K����Է�������Ϊ99�������ʽΪ____________
(5)д����˾ƥ��������NaOH��Һ��Ӧ�Ļ�ѧ����ʽ_________
(6)д����ͬʱ�������������İ�˾ƥ�ֵ������칹��Ľṹ��ʽ_______________
�ٱ�����ֻ������һ�ȴ���ں����Ȼ�����ˮ������ӡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ͼ��XΪ��Դ��YΪ������ʳ��ˮ�ͷ�̪��Һ����ֽ����ֽ�������һ��KMnO4��Һ��ͨ���Y������Ϻ�ɫɫ����d����ɢ�������ж���ȷ���ǣ�������
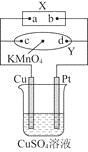
A.��ֽ��c�㸽������ɫ
B.Cu�缫������С��Pt�缫��������
C.�ձ�����Һ��pH�ȼ�С��������
D.�ձ���Һ��SO42-��Cu�缫�����ƶ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��CO2 �� CH4 �������Ʊ��ϳ�������������Ӧ����ʾ��ͼ���£�
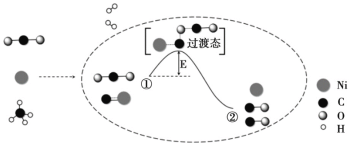
����˵������ȷ����
A.Ni �ڸ÷�Ӧ�������������뻯ѧ��Ӧ
B.�١�����������
C.�١�������̼�����Ķ��ѣ�����̼�������γ�
D.�ϳ�������Ҫ�ɷ�Ϊ CO �� H2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������Ԫ�ز�����ɵ����Ͳ������Ź㷺����;���ش��������⡣
(1)��̬��ԭ�Ӻ����________�ֲ�ͬ�ռ��˶�״̬�ĵ��ӡ������ܡ�����̬ԭ���У�����δ�ɶԵ��������ٵ�ԭ�Ӽ۲���ӹ����ʾʽ�������Ų�ͼ��Ϊ________��
(2)NiO��FeO�ľ���ṹ�������Ȼ��Ƶ���ͬ��Ni2+��Fe2+�����Ӱ뾶�ֱ�Ϊ69pm��74pm�����۵�NiO________FeO��������������������=������ԭ����________��
(3)Cr��һ�������ṹ��ͼ��ʾ��
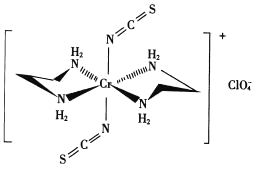
��������![]() �Ŀռ乹��Ϊ________�Ρ�
�Ŀռ乹��Ϊ________�Ρ�
���������У��������ӵ���λ��Ϊ_______��N������ԭ���γɵĻ�ѧ����Ϊ_______����
������H2NCH2CH2NH2���Ҷ�������̼ԭ�ӵ��ӻ���ʽ��________������������Ԫ�ص縺�ԴӴ�С��˳��Ϊ________��
(4)һ�����Ͳ��ϵľ����ṹ��ͼ1��ʾ��ͼ2�Ǿ�����Sm��Asԭ�ӵ�ͶӰλ�á�
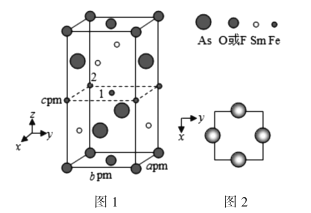
ͼ1��F��O��ͬռ�ݾ��������µ���λ�ã������ߵı���������x��1x��������û�����Ļ�ѧʽ��ʾΪ________�������ܶ���=________g��cm3���ú�x�ı���ʽ��ʾ���谢���ӵ�������ֵΪNA�����Ծ�������Ϊ��λ���Ƚ���������ϵ���Ա�ʾ�����и�ԭ�ӵ�λ�ã�����ԭ�ӷ������꣬����ͼ1��ԭ��1�����꣨![]() ������ԭ��2������Ϊ________��
������ԭ��2������Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Al2(SO4)3��MgSO4�Ļ����Һ�У��μ�NaOH��Һ�����ɳ������������NaOH��Һ�������ϵ����ͼ��ʾ����ԭ���Һ��Al2(SO4)3��MgSO4�����ʵ���Ũ��֮��Ϊ
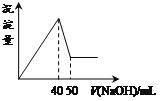
A.1:3B.1:2C.1:1D.2:1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����84����Һ����һ����NaClOΪ���ĸ�Ч�����������㷺���ڱ��ݡ����Ρ�ҽԺ��ʳƷ�ӹ���ҵ����ͥ�ȵ�����������ij��84����Һ��ƿ�岿�ֱ�ǩ��ͼ��ʾ������84����Һ��ͨ��ϡ��100��(���֮��)��ʹ�á���ش��������⣺
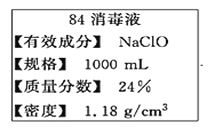
(1)NaClO����Ԫ�صĻ��ϼ�Ϊ________
(2)��ȡ100mL��Һ����˵��Ҫ��ϡ�ͺ�������������ϡ�ͺ����Һ��c(Na+)��______mol/L��
(3)��ͬѧ���Ķ�����84����Һ�����䷽������NaClO��������480 mL��NaClO��������Ϊ24%������Һ������ͼ��ʾ��������������Һ����ȱ�ٵIJ���������_____________��
(4)����ʱ������ȷ�IJ���˳����__________(����ĸ��ʾ��ÿ������ֻ��һ��)��
A.������ˮϴ���ձ�2�Ρ�3�Σ�ϴ��Һ��ע������ƿ����
B.���ձ��м�������ټ�ˮ�ܽ�
C.���ձ�������ȴ����Һ�ز�����ע������ƿ��
D.������ƿ�ǽ����������µߵ���ҡ��
E.���ý�ͷ�ιܼ�ˮ��ʹ��Һ��Һ��ǡ����̶�����
F.����������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶�1 cm��2 cm��
(5)��������������84����ҺŨ��ƫ�ߵ���___________(�����)��
A.û����ȴ�����¾�ת�Ʋ����ٶ���
B.ת��ʱû��ϴ���ձ���������
C.����ʱ����Һ��
D.����ʱ������ƽ������������
E.ҡ�Ⱥ���Һ����ڿ̶��ߣ��ּ�����ˮ���̶���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ͼ��ʾװ�òⶨþ����Ʒ�е���þ���������������������ᷴӦ���������壩
���������գ�
��1��������������Ŀ����_____
��2������a��������_____
��3��ȡ����þ����Ʒ�ֱ����ʵ�飬�������ݼ��±���
ʵ����� | þ��������g�� | ���������mL�����ѻ���ɱ�״���� |
1 | 0.053 | 44.60 |
2 | 0.056 | 47.05 |
����þ������������_____��������3λС����
��4������ⶨ���ƫ�ߣ����ܵ�ԭ����_____����ѡ���ţ�
a��װ��©��
b��δ��ȴ�����¼�����
c��þ���к�������þ
d��ĩ����ʱ�����ܵ�Һ�����ˮ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������I��һ����Ҫ��ҩ���м��壬����Robinson�ɻ���Ӧ�ϳ�I��·����ͼ���ش��������⣺

��֪��a.CH3COOCH3+CH3COOCH3![]()
b.
��1��A��һ�ֻ�������ֻ��һ�ֻ�ѧ������H����A�Ľṹ��ʽΪ__��C��ѧ����Ϊ__��
��2��C��D��F��G�ķ�Ӧ���ͷֱ�Ϊ__��__��
��3��D��E�Ļ�ѧ����ʽΪ__��
��4��H���������ŵ�����Ϊ__��I�Ľṹ��ʽΪ__��
��5��G�ж���ͬ���칹�壬�������������Ĺ���__�֣������������칹�������к˴Ź���������4���Ľṹ��ʽΪ__����дһ�֣���
�ٱ�������4��ȡ��������1molG������3molNaOH��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com