
����Ŀ����ˮ�DZ������Ȼ��Դ�����ú�ˮ���Եõ�һϵ�в�Ʒ��Ҳ���Խ��з���������
��1�������ȼҵ��Ʒ��������SO2��������������ͼ��ʾ��
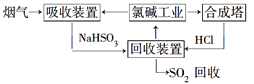
�١�����װ�á��з�����Ӧ�����ӷ���ʽ��__________________________��
������������ѭ�����õ�������________��
��2�����ú�ˮ���������Ч�ؽ��úȼ�չ������ŷŵ�SO2��ɵ�һϵ�л������⡣�乤��������ͼ��ʾ����Ȼ��ˮ���պ������������Ҫ���������������������䷴Ӧ�Ļ�ѧ����ʽ��_____________________��������ĺ�ˮ��Ҫ�����������ƣ���֮��Ϻ�����ŷš��ò�������ҪĿ����____________________��
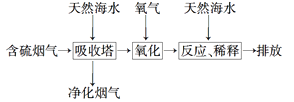
��3���Ӻ�ˮ���ᴿ���κ��ĸҺ�к���K����Na����Mg2���������ӡ���ĸҺ����һϵ�еļӹ����Ƶý���þ��
�ٴ����ӷ�Ӧ�ĽǶ�˼������ĸҺ�м���ʯ���������������___________��
��Ҫ����MgCl2��6H2O�Ƶ���ˮ�Ȼ�þ��Ӧ��ȡ�Ĵ�ʩ��________________��
�۵�����ڵ���ˮ�Ȼ�þ���õ�þ�������ض��Ļ�������ȴ��Ϊ����þ�����������п�������þ��������ȴ������________(����ĸ)��
A��Ar�� B��CO2 C������ D��O2 E��ˮ����
���𰸡�SO2��OH��=HSO3�� 2H2SO3��O2=2H2SO4 NaCl ʹ�������������ᷢ���кͷ�Ӧ ����Mg2��[����ȡMg(OH)2] ��HCl��������ˮ������MgCl2��ˮ�� A
��������
����(1) ���ȼҵ��Ӧ�Ļ�ѧ����ʽΪ��2NaCl+H2O![]() 2NaOH+H2��+Cl2��.��Һ����Ҫ�ɷ���NaOH������SO2������ͨ�����Һʱ������Ӧ��SO2��2NaOH=Na2SO3+H2O ��SO2����ʱ����SO2��NaOH=NaHSO3����Ӧ�����ӷ���ʽΪ��SO2��2OH��=SO32-+H2O��SO2��OH��=HSO3-����������ͼ���Կ�����������������ѭ�����õ�������NaCl����2�����������������������պ������������Ȼ��ˮ�ķ�Ӧԭ���Ļ�ѧ����ʽ��2H2SO3��O2=2H2SO4��������ĺ�ˮ�������ᣬˮ��Һ�����ԣ�������Ҫ�����������ƣ���֮��Ϻ�����ŷţ��ò�������ҪĿ����ʹ�������������ᷢ���кͷ�Ӧ����3������ĸҺ�м���ʯ���������������ʹMg2��ת��ΪMg(OH) 2������ȥ����MgCl2��ǿ�������Σ�����ʱ�λ������ڽᾧˮ�У��η���ˮ�ⷴӦ����Mg(OH) 2��HCl��HCl����ˮ�ֵ��������ӷ������õ�����Mg(OH) 2���塣Ԫ��Ҫ����MgCl2��6H2O�Ƶ���ˮ�Ȼ�þ��Ӧ��ȡ�Ĵ�ʩ����HCl��������ˮ��������MgCl2ˮ�⡣������Mg��Ժ�ǿ���ڸ���ʱ����������е�O2��ˮ����������Ӧ��Ҳ����CO2������Ӧ����MgO��C������Ҫ�ڶ�������Ar�Ļ�������ȴ��ѡ��ΪA��
2NaOH+H2��+Cl2��.��Һ����Ҫ�ɷ���NaOH������SO2������ͨ�����Һʱ������Ӧ��SO2��2NaOH=Na2SO3+H2O ��SO2����ʱ����SO2��NaOH=NaHSO3����Ӧ�����ӷ���ʽΪ��SO2��2OH��=SO32-+H2O��SO2��OH��=HSO3-����������ͼ���Կ�����������������ѭ�����õ�������NaCl����2�����������������������պ������������Ȼ��ˮ�ķ�Ӧԭ���Ļ�ѧ����ʽ��2H2SO3��O2=2H2SO4��������ĺ�ˮ�������ᣬˮ��Һ�����ԣ�������Ҫ�����������ƣ���֮��Ϻ�����ŷţ��ò�������ҪĿ����ʹ�������������ᷢ���кͷ�Ӧ����3������ĸҺ�м���ʯ���������������ʹMg2��ת��ΪMg(OH) 2������ȥ����MgCl2��ǿ�������Σ�����ʱ�λ������ڽᾧˮ�У��η���ˮ�ⷴӦ����Mg(OH) 2��HCl��HCl����ˮ�ֵ��������ӷ������õ�����Mg(OH) 2���塣Ԫ��Ҫ����MgCl2��6H2O�Ƶ���ˮ�Ȼ�þ��Ӧ��ȡ�Ĵ�ʩ����HCl��������ˮ��������MgCl2ˮ�⡣������Mg��Ժ�ǿ���ڸ���ʱ����������е�O2��ˮ����������Ӧ��Ҳ����CO2������Ӧ����MgO��C������Ҫ�ڶ�������Ar�Ļ�������ȴ��ѡ��ΪA��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ�˼���ij��Һ���Ƿ��г��������������ӣ�ij��ѧС���ͬѧ������������ʾ��ʵ����������м�������в�����������ʹʪ��ĺ�ɫʯ����ֽ�������ɸ�ʵ���ܵó�����ȷ�����ǣ� ��

A��ԭ��Һ��һ������SO42- B��ԭ��Һ��һ������NH4+
C��ԭ��Һ��һ������Cl- D��ԭ��Һ��һ������Fe3+
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����CO��H2��ɵĻ������2.4g��������O2�г��ȼ�պ����ɵ����в���ͨ��������Na2O2���壬Na2O2�������ӵ�����Ϊ
A. 1.2g B. 2.4 g C. 3.6![]() g D. ������
g D. ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪������Ksp(AgCl)��1.8��10��10��Ksp(AgBr)��5��10��13�������й�˵���������(�� ��)
A. �ڱ���AgCl��AgBr�Ļ����Һ�У�![]() ��360
��360
B. ��AgCl����Һ�еμ�ŨNaBr��Һ���������ɫ����
C. AgCl��ˮ���ܽ�ȼ�Ksp������NaCl��Һ�еĴ�
D. ����1 L NaCl��Һ��0.01 mol AgBrת��ΪAgCl����c(NaCl)��3.61 mol��L��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������Ϊ1L��pH������2������ʹ�����Һ�У���Ͷ��0.65gп��������ͼ��ʾ�ȽϷ��Ͽ���ʵ�ģ� ��

A. B. C. D.
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ����Ǧ����Ϊ��Դ��ģ���ȼҵ��ⱥ��ʳ��ˮ��װ��ͼ(C��D��Ϊʯī�缫)��
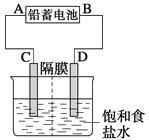
��֪��Ǧ�����ڷŵ�ʱ�������е缫��Ӧ��
����Pb��SO![]() ��2e��===PbSO4
��2e��===PbSO4
����PbO2��4H����SO![]() ��2e��===PbSO4��2H2O
��2e��===PbSO4��2H2O
(1)��д����ⱥ��ʳ��ˮ�Ļ�ѧ����ʽ_______________________________________��
(2)���ڵ�����C��һ��η�̪��Һ�����һ��ʱ���δ�ʺ�ɫ��˵��Ǧ���ص�A��Ϊ________����
(3)��Ǧ���ص��1 L����ʳ��ˮ��ʳ��ˮ������ʱ��
�����ռ���11.2 L(��״����)��������ҺpH=_________________��
����Ǧ��������H2SO4 2 mol������ռ���H2�����(��״����)Ϊ___________L��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ҵ������������Ҫ�ɷ���Al2O3��Fe2O3��SiO2���ᴿAl2O3��ұ������ԭ�ϣ���ȡ�IJ������̿�����������ͼ��ʾ��
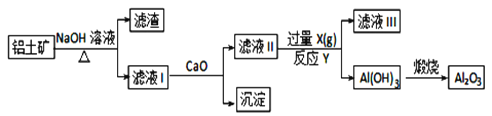
��֪��Na2SiO3 + Ca(OH)2= CaSiO3��+ 2NaOH
��1������������Һ��ȡ������ʱ��������Ӧ�����ӷ���ʽ�У�______________________��______________________��
��2����ҺII��ͨ�����������X��________����Ӧ�Ļ�ѧ����ʽ��____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������ƾ��壨Na2S2O3��5H2O��M=248 g��mol1����������Ӱ������ԭ�����ش��������⣺
��1����֪��Ksp(BaSO4)=1.1��1010��Ksp(BaS2O3)=4.1��105����������������г�������������ʣ�ѡ�������Լ����ʵ�鷽�����м��飺
�Լ���ϡ���ᡢϡH2SO4��BaCl2��Һ��Na2CO3��Һ��H2O2��Һ
ʵ�鲽�� | ���� |
��ȡ������Ʒ�������������ˮ | �ڹ�����ȫ�ܽ����ɫ������Һ |
��___________ | ��___________���д̼���������� |
�ݾ��ã�___________ | ��___________ |
��2������K2Cr2O7����Һ�����ⶨ��������ƵĴ��ȡ��ⶨ�������£�
����Һ���ƣ���ȡ1.2000 gij��������ƾ�����Ʒ��������в���ȴ������ˮ��__________���ܽ⣬��ȫ�ܽ��ȫ��ת����100 mL��_________�У�������ˮ��____________��
�ڵζ���ȡ0.00950 mol��L1��K2Cr2O7����Һ20.00 mL�������ữ��������KI��������Ӧ�� Cr2O72+6I+14H+![]() 3I2+2Cr3++7H2O��Ȼ���������������Ʒ��Һ�ζ���������ɫ��������Ӧ��I2+2S2O32
3I2+2Cr3++7H2O��Ȼ���������������Ʒ��Һ�ζ���������ɫ��������Ӧ��I2+2S2O32![]() S4O62+2I�����������Һ��Ϊָʾ���������ζ�������Һ__________����Ϊ�յ㡣ƽ�еζ�3�Σ���Ʒ��Һ��ƽ������Ϊ24.80 mL������Ʒ����Ϊ_________%������1λС������
S4O62+2I�����������Һ��Ϊָʾ���������ζ�������Һ__________����Ϊ�յ㡣ƽ�еζ�3�Σ���Ʒ��Һ��ƽ������Ϊ24.80 mL������Ʒ����Ϊ_________%������1λС������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͭ���ǹ�ҵ��ͭ����Ҫԭ�ϣ���Ҫ�ɷ�ΪCuFeS2����������ʯ��Ϊ�ⶨ�û�ͭ��Ĵ��ȣ�ijͬѧ���������ʵ�飺
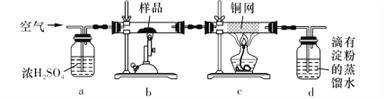
���õ�����ƽ��ȡ��ϸ�Ļ�ͭ����Ʒ1.150 g���ڿ��������½������գ�����Cu��Fe3O4��SO2���壬ʵ���ȡd����Һ��1/10������ƿ�У���0.05 mol L-1������Һ���еζ������ı�����Һ20. 00 mL����ش��������⣺
(1)����Ʒ��ϸ���ٽ��з�Ӧ����Ŀ����_____________________��������ҺӦʢ����___________(���ʽ������ʽ��)�ζ����С�
(2) aװ�õ�������__________________(����ĸ����)��
A.��ȥ�����еĶ�����̼
B.��ȥ�����е�ˮ����
C������������
D.�����ڹ۲졢���ƿ�������
(3)��ȥ��cװ�ã���ʹ�ⶨ���_______________(�ƫ�͡���ƫ�ߡ�����Ӱ�족)��д��Ӱ��ⶨ����Ļ�ѧ����ʽ��________________________��
(4)������Ӧ����������ͨһ��ʱ��Ŀ�������Ŀ����___________________________��
(5)ͨ�������֪���û�ͭ��Ĵ���Ϊ_________________________��
(6) ����ʵ���������ȷ����õĻ�ͭ����Ȼƫ�ͣ����ܵ�ԭ����Ҫ��_____________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com