
ij��ѧ��ȤС��ͬѧ���̡�֤�������д��ڵ�Ԫ�ء���ʵ��ʱ���Կα��ϵ����������������պ�Ļҽ��е�Ԫ����I-��ʽ���ڡ����������ʡ�����KI���屻�������ʣ���dz��ɫ����I2����KI����ֱ�Ӽ���ʱ��Ϊʲô���������أ���ˣ�С��ͬѧ���I-������������������֮���Ҫʲô����������ʵ��̽����
��������衿���ݾ���KI����ᱻ���������ʣ���Ͽ����ijɷ֣������ų� ��ϡ�������Ӱ�졣Ȼ���KI������������������裺
����һ����ҪH2O�IJ��룻
���������ҪCO2�IJ��룻
�������� ��
��ʵ����֤��
(1)Ϊ����֤�������С��ͬѧ���������ʵ�鷽����ȡ10mLKI��Һ(Ũ�Ƚϴ�)��5֧�Թ��У����Թ���ͨ��CO2������ߵμ����ᡣһ��ʱ���Ա��Թ��е���ɫ��dz��֮�����Թ��е��������Һ���ٴζԱ��Թ��е���ɫ��dz������ʵ��������±���
| �Թ���� | 1 | 2 | 3 | 4 | 5 |
| ͨ��CO2ʱ��/min | 0 | 1 | 5 | | |
| �����/�� | 0 | | | 3 | 6 |
| ��Һ����ɫ�Ա� | ��ɫ | dz��ɫ����ɫ�����μ�� | |||
| �μӵ�����Һ�����ɫ�Ա� | ��ɫ | dz��ɫ����ɫ�����μ�� | |||
| ʵ�鲽�� | Ԥ������ͽ��� |
| ��ȡһ�ݴ�����KI���壬�ֳ����ȷݣ� | |
| ��һ�ݼ��뵽װ�� �ļ���ƿA�У� һ�ݼ��뵽װ�� �ļ���ƿA�У� �ۼ����۲졣 | |
��13�֣�
[�������] N2��1�֣� �� CO2��H2O�Ĺ�ͬ���루2�֣���
[ʵ����֤] ��1�����ԣ�1�֣���4KI + O2 + 2CO2 ="=" 2K2CO3 + 2I2��3�֣�
��2��ʵ�鲽�裺 Ԥ������ͽ��� ��ȡһ�ݴ�����KI���壬�ֳ����ȷݣ� �ڸ����CO2��O2������壨1�֣�
��ʪ��CO2��O2������壨1�֣�������ƿA�е�KI������ɫ��ƣ�������KI���Բ���ҪH2O�μӣ�1�֣���
������ƿA�е�KI������ɫ���䣬������ƿB��KI������ɫ��ƣ�������KI����Ҫ��H2O�μӣ�1�֣���
�������������֣�
[�������] �ܷ��ҷ����ڸ��ﴦ��2�֣�
�����������������ı����Ƕ�����������̽����������Ӧ������Ϊ�����Ӻ�������������H2O��CO2�е�һ�ֻ����֣���̽���ķ������Χ��H2O��CO2��H2CO3���Ӷ����ʵ�����֤������ʵ������п������Ա�ʵ��Ͳ���ʵ�顣��ʵ����ƹ����У�����Ҫ�Ա�����H2O���뷴Ӧ��Ӧ������CO2��O2��CO2��O2��H2O�����鷴Ӧ���жԱȴӶ��õ����ۡ�
�ڽ��������Ҫע���������������α�֪ʶ�Ľ�ϣ����Խ����������Ļ������̽��н����������Ҫ���롢�������������������ȷ���淶��
���㣺������̽��ʵ��Ϊ����������Ԫ�ؼ�������֪ʶ��ʵ��̽���������������֪ʶ��


 Сѧѧϰ�ð���ϵ�д�
Сѧѧϰ�ð���ϵ�д� Сѧͬ�����������ܾ�ϵ�д�
Сѧͬ�����������ܾ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
����̼��ƹ㷺Ӧ���������ϡ���ֽ����ѧ���ġ���ī��Ϳ�ϡ��ܷ⽺�뽺ճ������ҵ����ŨCaCl2��Һ��ͨ��NH3��CO2�������Ƶ�����̼��ơ�ijУѧ��ʵ��С�������ͼ��ʾװ�ã���ȡ�ò�Ʒ��D��װ��պϡ�������֬�ޣ�ͼ�мг�װ������ȥ��
��.��ѡ�õ�ҩƷ�У�
a��ʯ��ʯ b�������Ȼ�����Һ c��6 mol/L���� d���Ȼ�� e����������
��1��A���Ʊ�����ʱ������ҩƷ�ǣ�ѡ����ĸ��ţ� ��
��2��B��ʢ�б���̼��������Һ���������� ��
��3��д����ȡ�����Ļ�ѧ����ʽ ��
��4����ʵ������У���C��ͨ�����������Ⱥ�˳��ģ�Ӧ��ͨ������Ļ�ѧʽ ��
��5������D���ڴ��Ƿ��а����ݳ��ķ����� ��
��6��д��������̼��ƵĻ�ѧ����ʽ ��
��7����ʵ��������а����ݳ���Ӧѡ������ װ�û��գ�����ţ���
�������������Ȼ����Ʒ�к�������̼�����ơ�Ϊ�˲ⶨ�Ȼ�淋�������������ѧ��ʵ��С�������������ʵ�����̣�
�Իش�
��1�������Լ�A�Ļ�ѧʽΪ
��2��B����������
��3����Ʒ���Ȼ�淋���������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
̼��þ������һ�����͵��������β����е���ǿ���ϡ�
��1���ϳɸ����ʵIJ������£�
����1������0.5mol��L-1MgSO4��Һ��0.5mol��L-1NH4HCO3��Һ��
����2������Ͳ��ȡ500mL NH4HCO3��Һ��1000mL�Ŀ���ƿ�У��������������¶ȿ�����50�档
����3����250mL MgSO4��Һ��μ���NH4HCO3��Һ�У�1min�ڵμ�����ð�ˮ������ҺpH��9.5��
����4������1h���ˣ�ϴ�ӡ�
����5����40�����ո������и���10h����̼��þ�����Ʒ��MgCO3��nH2O n=1~5����
�ٲ���2�����¶���50�棬�Ϻõļ��ȷ����� ��
�ڲ���3����MgCO3��nH2O���������ӷ���ʽΪ ��
�۲���4�����Ƿ�ϴ�Ӹɾ��ķ����� ��
��2���ⶨ�ϳɵ�MgCO3��nH2O�е�nֵ��
����1.000g̼��þ���룬������ͼ��ʾ�Ĺ��ƿ�м���ˮ����ϡ�����뾧�뷴Ӧ�����ɵ�CO2��NaOH��Һ���գ��������·�Ӧ4~5h����Ӧ���ڽ��¶�����30�棬�����ձ��е���Һ����֪Ũ�ȵ�����ζ������CO2���������ظ���������2�Ρ�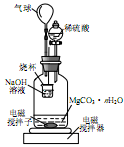
��ͼ������������� ��
��������Ӧ����Ҫ���µ�30�棬��ҪĿ���� ��
����3��ʵ����ÿ1.000g̼��þ���������CO2ƽ��ֵΪa mol����nֵΪ ���ú�a�ı���ʽ��ʾ����
��3����ȡ100g���������Ʒ�������ط���������������ͼ��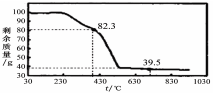
��������ºϳɵľ����У�n= ��ѡ�1��2��3��4��5����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ͭ���ǹ�ҵ��ͭ����Ҫԭ�ϣ�����Ҫ�ɷ�ΪCuFeS2������һ����Ȼ��ͭ������SiO2����Ϊ�˲ⶨ�û�ͭ��Ĵ��ȣ�ijͬѧ���������ʵ�飺
�ֳ�ȡ��ϸ�Ļ�ͭ����Ʒ1.150g���ڿ��������½������գ�����Cu��Fe3O4��SO2���壬ʵ���ȡd����Һ�� ������ƿ�У���0.05mol/L������Һ���еζ������ı���Һ20.00mL����ش��������⣺
������ƿ�У���0.05mol/L������Һ���еζ������ı���Һ20.00mL����ش��������⣺
��1��������Ʒ���õ�����Ϊ_____���������ƽ��������ƽ����������Ʒ��ϸ���ٷ�Ӧ����Ŀ����_______ ��
��2��װ��a��c�����÷ֱ���____��____�����ţ���
a����ȥSO2����
b����ȥ�����е�ˮ����
c��������������
d�������ڹ۲��������
e����ȥ��Ӧ����������
��3��������Ӧ����������ͨһ��ʱ��Ŀ�������Ŀ����___________��
��4��ͨ�������֪���û�ͭ��Ĵ���Ϊ________��
��5��������ͼװ���������ʵ��װ��d��ͬ�����Դﵽʵ��Ŀ�ĵ���____������ţ���
��6������ԭװ��d�е���Һ��ΪBa(OH)2����õĻ�ͭ�����Ϊ��1%������ʵ���������ȷ�����ܵ�ԭ����Ҫ��_____________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
CuSO4��Һ��Na2CO3��Һ��ϲ�������ɫ������������ij��ȤС��Գ�����ɵ�̽����
��������衿
����1������ΪCu (OH)2
����2������Ϊ
����3������Ϊ��ʽ̼��ͭ[��ѧʽ�ɱ�ʾΪnCuCO3��mCu (OH)2]
���������ϡ���������һ�ֳ������Ⱦ��ֽ⣨����������ᾧˮ����
������̽����
����1������������Һ���ˣ�������ˮϴ�ӣ�������ˮ�Ҵ�ϴ�ӣ���ɣ�
����2����ͬѧȡһ�������壬�����������õ�����װ�ã��г�����δ���������ж���ʵ�飻
��1������Ӧ��A������ɫ�����ڣ�C������������֤������ ������
��2����ͬѧ��ΪֻҪ����ͼ��Bװ�õ��Լ���������ij�Լ������֤�������м��裬���Լ��� ������ţ���
a��Ũ���� b����ˮCuSO4 c����ʯ�� d��P2O5
��3����ͬѧ����B�Լ�����֤����3������ʵ�������� ��
������̽����
��4����ͬѧ��һ��̽������3�й������ɣ�
����ͬѧ���һЩ������20������ݣ����±�����C�еij���ʯ��ˮ��ΪBa(OH)2��Һ����ԭ���� ��˫ѡ������ţ�
| �ܽ��(S)/g | �ܶȻ�(Ksp) | Ħ������(M)/g��mol��1 | |||
| Ca(OH)2 | Ba(OH)2 | CaCO3 | BaCO3 | CaCO3 | BaCO3 |
| 0.16 | 3.89 | 2.9��10-9 | 2.6��10-9 | 100 | 197 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
S2Cl2�ǹ�ҵ�ϳ��õ�����ʵ�����Ʊ�S2Cl2�ķ�����2�֣�
�� CS2+3Cl2 CCl4+S2Cl2���� 2S+Cl2
CCl4+S2Cl2���� 2S+Cl2 S2Cl2��
S2Cl2��
��֪S2Cl2����Ԫ����+1�ۣ�����ʽ�� �������ȶ�����ˮ������᪻���Ӧ��һ������Ԫ�ؼ�̬���ߣ�һ���ֽ��ͣ�����Ӧ�漰�ļ������ʵ��۷е����£�
�������ȶ�����ˮ������᪻���Ӧ��һ������Ԫ�ؼ�̬���ߣ�һ���ֽ��ͣ�����Ӧ�漰�ļ������ʵ��۷е����£�
| ���� | S | CS2 | CCl4 | S2Cl2 |
| �е�/�� | 445 | 47 | 77 | 137 |
| �۵�/�� | 113 | -109 | -23 | -77 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijУ��ѧС���ͬѧ������̼��Ϊ�缫����Ȼ�ͭ��Һʱ��������̼���ϳ����к�ɫ���������⣬����������ɫ����������Ϊ̽������̼���ϵIJ����������¹��̣�
�������
ͭ�Ļ�������ɫ�������£�
| ���� | ��ɫ������ | ���� | ��ɫ������ |
| ������ͭCu(OH)2 | ��ɫ���岻����ˮ | ����ͭ��CuSO4�� | ��Һ����ɫ |
| ������ͭ��CuO�� | ��ɫ���岻����ˮ | �Ȼ�ͭ��CuCl2�� | ��Һ����ɫ��ϡ��Һ����ɫ |
| �Ȼ���ͭ��CuCl�� | ��ɫ���岻����ˮ | ��ʽ�Ȼ�ͭ | ��ɫ���岻����ˮ |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
Ư����һ�ֳ��õ���������
��1����ҵ������Ư�۷�Ӧ�Ļ�ѧ����ʽΪ��________________ __��Ư�۵���Ч�ɷ�Ϊ ��
��2��ij̽��С����г��Ϲ�����һ����װ�����Ư�ۣ��Ը�Ư�۵ijɷֽ���̽�������������Լ������ʵ�鷽��������ʵ�顣���ڴ�������ʵ�鱨�档
��ѡ�Լ���2mol��L��1NaOH��Һ��2mol��L��1HCl��Һ��2mol��L��1HNO3��Һ��0.5mol��L��1BaCl2��Һ��0.01mol��L��1AgNO3��Һ������ʯ��ˮ��ʯ����Һ����̪��Һ������ˮ��
| ʵ�鲽�� | Ԥ����������� |
| ����1��ȡ����Ư��������������ˮ����ֽ��裬���ã����ˣ��ó�������Һ�� | |
| ����2���������������2mol��L��1HCl��Һ��������������ͨ�� | ���� ���ۣ� |
| ����3��ȡ��Һ��װA��B��֧�Թܡ���A�Թܣ� | ������Һ�ȱ��ɫ��Ȼ����ɫ�� ���ۣ� |
| ����4����B�Թܣ� | ��������ɫ������ ���ۣ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
ʵ�����Ʊ����ռ������SO2�������������¡�װ�ã�����SO2���������������Ӹ�������˳��Ϊ��ӣ� ���ӣ� ���ӣ� ���ӣ� ���ӣ棨��ӿ���ĸ�� �� ��
| A��b c d e | B��d e b c | C��d e c b | D��e d b c |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com