
(12��)���������������ЧӦ����Դ��ȱ����������ӣ���ν��ʹ�����CO2�ĺ�������Ч�ؿ�������CO2�������˸������ձ����ӡ�
��1��Ŀǰ���ó��ٽ�CO2����״̬������̬��Һ̬֮�䣩���������������ѳ�Ϊһ�����ƣ���һ�����Ի����Ļ����������� ��
��2����CO2ת�����л������Чʵ��̼ѭ����CO2ת�����л�������Ӻܶ࣬�磺
���Ϸ�Ӧ�У�����ܵ��� ��ԭ����������ߵ��� ��
��3��Ϊ̽����CO2������ȼ�ϼ״��ķ�Ӧԭ�����ֽ�������ʵ�飺
�����Ϊ1L���ܱ������У�����1molCO2��3molH2��һ�������·�����Ӧ�� CO2��g��+3H2��g�� CH3OH��g��+H2O��g����H=-49.0kJ/mol
CO2��g��+3H2��g�� CH3OH��g��+H2O��g����H=-49.0kJ/mol
���CO2��CH3OH��g����Ũ����ʱ��仯��ͼ��ʾ��
�ٴӷ�Ӧ��ʼ��ƽ�⣬������ƽ����Ӧ����v��H2��= mol/��L��min��
�ڸ÷�Ӧ��ƽ�ⳣ������ʽΪ �������¶ȣ�ƽ�ⳣ������ֵ��
�����������С�����䡱����
�����д�ʩ����ʹn��CH3OH��/n��CO2��������� .
| A�������¶� | B������He��g����ʹ��ϵѹǿ���� |
| C����H2O��g������ϵ�з��� | D���ٳ���1molCO2��3molH2 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
(12��)���������������ЧӦ����Դ��ȱ����������ӣ���ν��ʹ�����CO2�ĺ�������Ч�ؿ�������CO2�������˸������ձ����ӡ�
��1��Ŀǰ���ó��ٽ�CO2����״̬������̬��Һ̬֮�䣩���������������ѳ�Ϊһ�����ƣ���һ�����Ի����Ļ����������� ��
��2����CO2ת�����л������Чʵ��̼ѭ����CO2ת�����л�������Ӻܶ࣬�磺
���Ϸ�Ӧ�У�����ܵ��� ��ԭ����������ߵ��� ��
��3��Ϊ̽����CO2������ȼ�ϼ״��ķ�Ӧԭ�����ֽ�������ʵ�飺
�����Ϊ1L���ܱ������У�����1molCO2��3molH2��һ�������·�����Ӧ��
 CO2��g��+3H2��g�� CH3OH��g��+H2O��g����H=-49.0kJ/mol
CO2��g��+3H2��g�� CH3OH��g��+H2O��g����H=-49.0kJ/mol
���CO2��CH3OH��g����Ũ����ʱ��仯��ͼ��ʾ��
�ٴӷ�Ӧ��ʼ��ƽ�⣬������ƽ����Ӧ����v��H2��= mol/��L��min��
�ڸ÷�Ӧ��ƽ�ⳣ������ʽΪ �������¶ȣ�ƽ�ⳣ������ֵ��
�����������С�����䡱����
�����д�ʩ����ʹn��CH3OH��/n��CO2��������� .
A�������¶� B������He��g����ʹ��ϵѹǿ����
C����H2O��g������ϵ�з��� D���ٳ���1molCO2��3molH2
��4�������Ǻϳɰ�����Ҫԭ�ϣ��ϳɰ���Ӧ���Ȼ�ѧ����ʽ���£�
�ٵ��ϳɰ���Ӧ�ﵽƽ��ı�ijһ������������ı�N2��H2��NH3����������Ӧ������ʱ��Ĺ�ϵ����ͼ��ʾ��
ͼt1ʱ����ƽ���ƶ������������� ��
���б�ʾƽ��������NH3������ߵ�һ��ʱ���� ��
���¶�ΪT��Cʱ����3amolH2��amolN2������л������ܱ������У���������������ƶ�����ַ�Ӧ����N2��ת����Ϊ50%���������ͬ�¶��½�3amolH2��amolN2��2amolNH3�������������У�ƽ��ʱH2��ת����Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꽭��ʡ�߰���ѧ�߶���ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ������
����12�֣��������������ЧӦ����Դ��ȱ����������ӣ���ν��ʹ�����CO2�ĺ�������Ч�ؿ�������CO2�������˸������ձ����ӡ�
��.Ŀǰ��ҵ����һ�ַ�������CO2������ȼ�ϼ״���Ϊ̽���÷�Ӧԭ������������ʵ�飬�����Ϊ1L���ܱ������У�����1mol CO2��3.25 mol H2����һ�������·�����Ӧ���ⶨCO2��CH3OH(g)��H2O(g)��Ũ����ʱ��仯����ͼ��ʾ��
��1���ӷ�Ӧ��ʼ��ƽ�⣬������ƽ����Ӧ���� �� mol/(L��min)
�� mol/(L��min)
��2����������CO2��ת����Ϊ ��
��3�����д�ʩ����ʹn(CH3OH)/n(CO2)������� ��
| A�������¶� | B������ʱ���뵪�� |
| C����ˮ��������ϵ�з��� | D���ø���Ч�Ĵ��� |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�콭��ʡ�߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ������
����12�֣��������������ЧӦ����Դ��ȱ����������ӣ���ν��ʹ�����CO2�ĺ�������Ч�ؿ�������CO2�������˸������ձ����ӡ�
��.Ŀǰ��ҵ����һ�ַ�������CO2������ȼ�ϼ״���Ϊ̽���÷�Ӧԭ������������ʵ�飬�����Ϊ1L���ܱ������У�����1mol CO2��3.25 mol H2����һ�������·�����Ӧ���ⶨCO2��CH3OH(g)��H2O(g)��Ũ����ʱ��仯����ͼ��ʾ��

��1���ӷ�Ӧ��ʼ��ƽ�⣬������ƽ����Ӧ����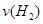 �� mol/(L��min)
�� mol/(L��min)
��2����������CO2��ת����Ϊ ��
��3�����д�ʩ����ʹn(CH3OH)/n(CO2)������� ��
A�������¶� B������ʱ���뵪��
C����ˮ��������ϵ�з��� D���ø���Ч�Ĵ���
��.��ͼ��һ���Ҵ�ȼ�ϵ�ع���ʱ��ʾ��ͼ���ҳ��е������缫һ����ʯī�缫��һ�������缫������ʱM��N�����缫�������������٣���ش��������⣺

��1��M���ĵ缫����Ϊ ��
��2�������Ҵ��IJ��缫�ĵ缫��ӦʽΪ ��
��3���ڴ˹������ҳ���ijһ�缫����������4.32gʱ����ʱ�ҳ���Һ�����Ϊ400mL�����ҳ�����Һ��pHΪ ��
��4�����ڳ��³�ѹ�£�1g C2H5OHȼ������CO2��Һ̬H2Oʱ�ų�29.71kJ��������ʾ�÷�Ӧ���Ȼ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��09-10�꽨�����и߶���ѧ����ĩ���Ի�ѧ�� ���ͣ������
(12��)���������������ЧӦ����Դ��ȱ����������ӣ���ν��ʹ�����CO2�ĺ�������Ч�ؿ�������CO2�������˸������ձ����ӡ�
��1��Ŀǰ���ó��ٽ�CO2����״̬������̬��Һ̬֮�䣩���������������ѳ�Ϊһ�����ƣ���һ�����Ի����Ļ����������� ��
��2����CO2ת�����л������Чʵ��̼ѭ����CO2ת�����л�������Ӻܶ࣬�磺
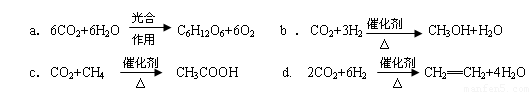
���Ϸ�Ӧ�У�����ܵ��� ��ԭ����������ߵ��� ��
��3��Ϊ̽����CO2������ȼ�ϼ״��ķ�Ӧԭ�����ֽ�������ʵ�飺
�����Ϊ1L���ܱ������У�����1molCO2��3molH2��һ�������·�����Ӧ��
 CO2��g��+3H2��g�� CH3OH��g��+H2O��g����H=-49.0kJ/mol
CO2��g��+3H2��g�� CH3OH��g��+H2O��g����H=-49.0kJ/mol
���CO2��CH3OH��g����Ũ����ʱ��仯��ͼ��ʾ��
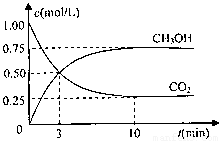
�ٴӷ�Ӧ��ʼ��ƽ�⣬������ƽ����Ӧ����v��H2��= mol/��L��min��
�ڸ÷�Ӧ��ƽ�ⳣ������ʽΪ �������¶ȣ�ƽ�ⳣ������ֵ��
�����������С�����䡱����
�����д�ʩ����ʹn��CH3OH��/n��CO2��������� .
A�������¶� B������He��g����ʹ��ϵѹǿ����
C����H2O��g������ϵ�з��� D���ٳ���1molCO2��3molH2
��4�������Ǻϳɰ�����Ҫԭ�ϣ��ϳɰ���Ӧ���Ȼ�ѧ����ʽ���£�
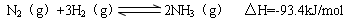
�ٵ��ϳɰ���Ӧ�ﵽƽ��ı�ijһ������������ı�N2��H2��NH3����������Ӧ������ʱ��Ĺ�ϵ����ͼ��ʾ��
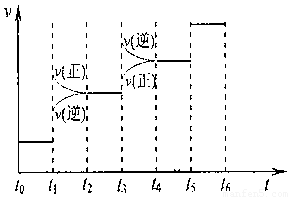
ͼt1ʱ����ƽ���ƶ������������� ��
���б�ʾƽ��������NH3������ߵ�һ��ʱ���� ��
���¶�ΪT��Cʱ����3amolH2��amolN2������л������ܱ������У���������������ƶ�����ַ�Ӧ����N2��ת����Ϊ50%���������ͬ�¶��½�3amolH2��amolN2��2amolNH3�������������У�ƽ��ʱH2��ת����Ϊ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com