
��������(NaClO2)��һ��ǿ������Ư�����㷺���ڷ�֯��ӡȾ��ʳƷ��ҵ�����ڼ��Ի������ȶ����ڡ�ijͬѧ�������Ϻ��������NaClO2����Ҫ�������¡�
(1)˫��ˮ�ĽṹʽΪ____________�����з�����Ӧ�Ļ�ԭ����__________(�ѧʽ)��
(2)���з�Ӧ�����ӷ���ʽ��_______________________________________��
(3)A�Ļ�ѧʽ��________��װ�â���A��________����������
(4)ClO2��һ�ָ�Чˮ�������������������ƺ�ϡ����Ϊԭ���Ʊ���
��д���÷�Ӧ�Ļ�ѧ����ʽ��__________________________________________��
���о�����������Ӧ��ʼʱ����Ũ�Ƚϴ��������������Cl2�������ӷ���ʽ���Ͳ���Cl2��ԭ��__________________________________________��
(5)NaClO2���ʿɷֽ�ΪNaClO3��NaCl��ȡ������NaClO2������һ�ݸ����ʵ�����ʹ֮���ʣ���һ���ϸ棬�������Һ�����ֱ�������FeSO4��Һ��Ӧʱ������Fe2�������ʵ���________(���ͬ��������ͬ�������жϡ�)��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��������ˮ�õ���ˮ����������ˮ�õ���ˮ�����й������Ƶİ�ˮ����ˮ��������ȷ����
| A������ˮ�����ǻ�����Һ�к��е��������ࡢ��Ŀ��ͬ |
| B������ˮ���ж����ڿ��淴Ӧ�Ļ�ѧƽ���������ʵĵ���ƽ�� |
| C������ˮ�����д̼�����ζ������Ư���л�ɫ�� |
| D������ˮ������ʱ��Ͼú���Ϊ��ͬ��ԭ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������һ���ḻ���ʱ��⣬ͨ����ˮ���ۺ����ÿɻ���������ʹ�����ʹ�á�
��1����ˮ���εĿ������ã�
�ٺ�ˮ����Ŀǰ�����Ϊ�������������ѡ��Զ�뽭���뺣�ڣ�������꣬��ϫ��������ƽ̹�տ��ĺ�̲�����������Ϊ��ˮ�ء������غ� �ء�
��Ŀǰ��ҵ�ϲ��ñȽ��Ƚ������ӽ���Ĥ���۷������ȼҵ�������ڵ����������ӽ���Ĥֻ����������ͨ������ֹ�����Ӻ�����ͨ������˵���ȼ������������ӽ���Ĥ�����ã� ��дһ�㼴�ɣ���
��2�����������ǽ��귢չ���һ�ֽϺõĺ�ˮ������������ԭ����ͼ��ʾ�����о���ѡ���Ե������ӽ���Ĥ�������ӽ���Ĥ������С���ش���������⣺
�ٺ�ˮ����ֱ��ͨ�뵽�������У������� ��
��A���ų����� �����ˮ����Ũˮ������
��3���ÿ�±����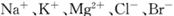 �����ӣ�����ȡ�壬�������������£�
�����ӣ�����ȡ�壬�������������£�
�����������е���Һ��BrO3-�����������з�Ӧ�����ӷ���ʽΪ�� ��
��ͨ�����Ȼ��ѻ�ú�Br2����Һ��Ϊ�λ��辭�����������ա��ữ���»�ú�Br2����Һ�� ��
������������ͨ��ˮ�������ȣ������¶���90�����ҽ��������ԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(7��)���ᡢ�������������ѧ�γ����ġ������ᡱ���־������������ͭ��Ӧ��������ش��������⣺
��1��ϡ�����Cu��Ӧ������ϡ�����м���H2O2����������������������ʱ��ԭ����Ϊˮ�������ʹͭ˳���ܽ⡣�÷�Ӧ�Ļ�ѧ����ʽΪ��__________________________________��
��2����һ�������18 mol��L-1��Ũ�����м������ͭƬ������ʹ֮��Ӧ������ԭ������Ϊ0.9mol����Ũ�����ʵ�����_________(����ڡ��������ڡ���С�ڡ�)100mL����ʹʣ���ͭƬ�����ܽ⣬�������м�����������Һ����KNO3��Һ������÷�Ӧ�����ӷ���ʽΪ______________��
��3��������ͼ�����������ƶ���XΪ_______(�����)��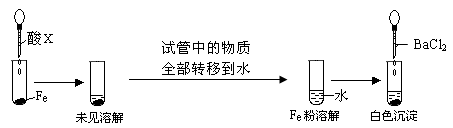
a��Ũ���� b��Ũ���� c��Ũ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(8��)
��Ԫ�ص��⻯����������ڹ�ҵ�������������ж��й㷺Ӧ�ã��ش��������⣺
(1)��Ԫ��ԭ�ӵ�L�������Ϊ ��
(2)NH3��NaClO��Ӧ�ɵõ���(N2H4)���÷�Ӧ�Ļ�ѧ����ʽΪ ��
(3)�¿���Ϊ�����������ȼ�ϣ���������N2O4��Ӧ����N2��ˮ������
��֪����N2(g)+2O2(g)= N2O4 (1) ��H1= -19.5kJ��mol-1
��N2H4 (1) + O2(g)= N2(g) + 2 H2O(g) ��H2= -534.2kJ��mol-1
д���º�N2O4��Ӧ���Ȼ�ѧ����ʽ ��
(4)��һ����ȼ�ϵ����һ�ּ��Ե�أ��õ�طŵ�ʱ�������ķ�ӦʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������Ҫ�İ뵼����ϣ��������ִ����ӹ�ҵ�Ļ������ش��������⣺
��1����̬Siԭ���У�����ռ�ݵ�����ܲ���� �����ܲ���е�ԭ�ӹ����Ϊ ��������Ϊ ��
��2������Ҫ�Թ����Ρ� �Ȼ��������ʽ�����ڵؿ��С�
��3�����ʹ��������ʯ�ṹ���Ƶľ��壬����ԭ����ԭ��֮���� ���ϣ��侧���й���8��ԭ�ӣ�����������λ�ù��� ��ԭ�ӡ�
��4�����ʹ��ͨ������(SiH4)�ֽⷴӦ���Ʊ�����ҵ�ϲ���Mg2Si��NH4CI��Һ�������з�Ӧ�Ƶ�SiH4���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��5��̼����йػ�ѧ������������ʾ����Ҫ�����ͽ��������й���ʵ��
| ��ѧ�� | C-C | C-H | C-O | Si-Si | Si-H | Si-O |
| ���ܣ�KJ/mol�� | 356 | 413 | 336 | 226 | 318 | 452 |
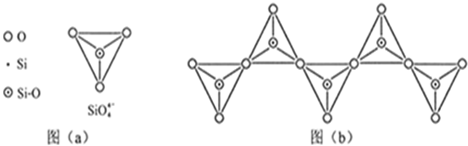
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪A��B��C��D��E��F��G��H���Է�������ͼ��ʾ��ת������Ӧ�в�������������ȥ�����У�A��GΪͬһ����Ԫ�صĵ��ʣ�B��C��H��ͨ�������Ϊ���壬������C��һ���γ�����Ĵ�����Ⱦ�
����գ�
(1)�E��������; �� ��
(2)��Ӧ�ڵ����ӷ���ʽΪ ��
(3)��Ӧ�۵Ļ�ѧ����ʽ�� ��
(4)��Ӧ�ܵ����ӷ���ʽ�� ��
(5)д��һ����A����H���û���Ӧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ijѧУ��ѧѧϰС��Ϊ̽���������������ʣ�����ͼ��ʾװ�ý���ʵ�顣
��ش��������⣺
(1)װ�ü���ʢ��Ũ���������A�������� ����װ���з�����Ӧ�Ļ�ѧ����ʽΪ ��
(2)ʵ������У�װ���ҡ����г��ֵ�����ֱ��� ��װ�ñ��е��Թ��ڷ�����Ӧ�����ӷ���ʽΪ ��
(3)Ϊ��̽��NO�Ļ�ԭ�ԣ�������װ�ö��ĵ�����C��ͨ��һ�����壬ͨ������������������ ��
(4)ȡ��װ�ñ��е��Թ�D�������еμ�FeSO4��Һ����Һ��Ϊ ɫ��Ϊ��֤����Ԫ���ڸ÷�Ӧ�еIJ������������Һ�еμ�KSCN��Һ����Һ��Ϊ ɫ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����þ����[Mg(ClO3)2��6H2O]���������ջ�ǰ��Ҷ����С�����������ݼ����������ʵ�����Ʊ�����Mg(ClO3)2�IJ����������£�
(1)�Ʊ�NaClO3���壺��ʵ�����������ͼ��ʾװ����ȡNaClO3��ͼ�У�aΪ��������װ�ã�bΪNaClO3���Ʊ�װ�ã�cΪβ������װ�á�
��װ��a�з�Ӧ�����ӷ���ʽΪ____________________________
�ڹ�ҵ����ʯ��������ռ���Һ����β����ԭ����___________________________
��װ��b�з�����Ӧ�Ļ�ѧ����ʽΪ3Cl2��6NaOH 5NaCl��NaClO3��3H2O�����Ʋ��ڼ���NaClO��Һʱ������Ӧ�Ļ�ѧ����ʽ��______________________________��
5NaCl��NaClO3��3H2O�����Ʋ��ڼ���NaClO��Һʱ������Ӧ�Ļ�ѧ����ʽ��______________________________��
(2)�Ʊ�����þ���壺�������ᴿ�Ƶõ�NaClO3��MgCl2����ѧ��Ӧ����ʽ������֮�Ȼ�Ͽ��Ƶ�Mg(ClO3)2��ԭ��ΪMgCl2��2NaClO3=Mg(ClO3)2��2NaCl����֪���ֻ�������ܽ��(S)���¶�(T)�仯��������ͼ��ʾ��
�벹����(1)�Ƶõ�NaClO3�Ʊ�Mg(ClO3)2��6H2O�IJ������裺
�ٰ�������֮�Ƚ�MgCl2��NaClO3�������85 �����ˮ�У�����������
��______________________��
��______________________��
���ؽᾧ��
(3)�������ۣ�����60 �� Na2CO3��Һ����Cl2Ҳ���Ƶ�NaClO3����д���÷�Ӧ�Ļ�ѧ����ʽ��__________________________________________��
���Ʊ�����þ����ʱ�������ؽᾧ������Ŀ����___________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com