
������Ҫ�İ뵼����ϣ��������ִ����ӹ�ҵ�Ļ������ش��������⣺
��1����̬Siԭ���У�����ռ�ݵ�����ܲ���� �����ܲ���е�ԭ�ӹ����Ϊ ��������Ϊ ��
��2������Ҫ�Թ����Ρ� �Ȼ��������ʽ�����ڵؿ��С�
��3�����ʹ��������ʯ�ṹ���Ƶľ��壬����ԭ����ԭ��֮���� ���ϣ��侧���й���8��ԭ�ӣ�����������λ�ù��� ��ԭ�ӡ�
��4�����ʹ��ͨ������(SiH4)�ֽⷴӦ���Ʊ�����ҵ�ϲ���Mg2Si��NH4CI��Һ�������з�Ӧ�Ƶ�SiH4���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��5��̼����йػ�ѧ������������ʾ����Ҫ�����ͽ��������й���ʵ��
| ��ѧ�� | C-C | C-H | C-O | Si-Si | Si-H | Si-O |
| ���ܣ�KJ/mol�� | 356 | 413 | 336 | 226 | 318 | 452 |
��1��M��9��4 ��2���������裻 ��3�����ۼ���3
��4��Mg2Si��4NH4Cl=SiH4��4NH3��2MgCl2
��5���ٹ����е�Si��Si����Si��H���ļ���С������������C��C����C��H���ļ��ܣ��ȶ��Բ���ѣ����³������������γɣ����Թ���������������϶�Զ���������ࡣ
�����ڼ���Խ������Խ�ȶ���C��H���ļ��ܴ���C��O���ļ��ܣ���C��H����C��O���ȶ�����Si��H���ļ���ȴԶС��Si��O���ļ��ܣ�����Si��H�����ȶ������������γ��ȶ��Ը�ǿ��Si��O�������������������
��6��sp3��1:3��[SiO3]n2n��(��SiO32��)
����


 ���ƿ�����ϵ�д�
���ƿ�����ϵ�д� ���¿쳵����������ϵ�д�
���¿쳵����������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
����ͭ���Ʊ�Cu��Zn��Alϵ��������Ҫԭ�ϡ�����������ȡ����ͭ��ʵ�鷽���ɹ�ѡ�ã���ͭ��ϡ���ᷴӦ��ȡ��3Cu + 8HNO3��ϡ�� 3Cu��NO3��2 + 2NO��+ 4H2O
3Cu��NO3��2 + 2NO��+ 4H2O
��ͭ��Ũ���ᷴӦ��ȡ��Cu + 4HNO3��Ũ��= Cu��NO3��2 + 2NO2��+ 2H2O
�����Ƚ�ͭм�ڿ����м�����������ͭ������ͭ��ϡ���ᷴӦ��ȡ��2Cu + O2  2CuO��CuO + 2HNO3 = Cu(NO3)2 + H2O �����й�˵����ȷ���ǣ� ��
2CuO��CuO + 2HNO3 = Cu(NO3)2 + H2O �����й�˵����ȷ���ǣ� ��
| A����ȡ����������ͭ����������������� |
| B����ȡ����������ͭ���ڲ������ж�����Ȣ��� |
| C�����ַ����У��ڢ۷�����û��� |
| D�����ַ����ķ�Ӧ�����������������н��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ѧ��ѡ��2����ѧ�뼼����(15��)
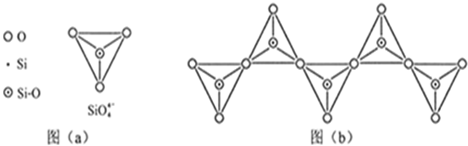
��ʯ��Ҫ������ơ�Ca3(PO4)2��H2O������ʯ��Ca3(OH)(PO4)3������ʽ���ڡ�ͼ(a)ΪĿǰ��������ʯ���õĴ������������ʪ��������ָ��ʯ�ù�������ֽ��Ʊ����ᡣͼ(b)���ȷ�������������������ʯ�Ƶ��������̡�

�������ʵ�����������£�
| | �۵�/�� | �е�/�� | ��ע |
| ���� | 44 | 280.5 | |
| PH3 | -133.8 | -87.8 | ������ˮ�����л�ԭ�� |
| SiF4 | -90 | -86 | ��ˮ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ��ʾ�ɶ�����Ԫ����ɵ�һЩ����֮���ת����ϵ��ijЩ��Ӧ��������ȥ�����������ʾ�йص�һ�ַ�Ӧ��������ijЩ�����Ѿ���ȥ��������A��B��D�ڳ����¾�Ϊ��ɫ�̼�����ζ�����壬C��ʹʪ��ĺ�ɫʯ����ֽ���������壬M���������ɫҺ�塣
�� ����F�Ļ�ѧʽ ��
�� ����B�ĵ���ʽ ��
�� д��C��E�Ļ�ѧ����ʽ ��
�� д��G��E�����ӷ���ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������(NaClO2)��һ��ǿ������Ư�����㷺���ڷ�֯��ӡȾ��ʳƷ��ҵ�����ڼ��Ի������ȶ����ڡ�ijͬѧ�������Ϻ��������NaClO2����Ҫ�������¡�
(1)˫��ˮ�ĽṹʽΪ____________�����з�����Ӧ�Ļ�ԭ����__________(�ѧʽ)��
(2)���з�Ӧ�����ӷ���ʽ��_______________________________________��
(3)A�Ļ�ѧʽ��________��װ�â���A��________����������
(4)ClO2��һ�ָ�Чˮ�������������������ƺ�ϡ����Ϊԭ���Ʊ���
��д���÷�Ӧ�Ļ�ѧ����ʽ��__________________________________________��
���о�����������Ӧ��ʼʱ����Ũ�Ƚϴ��������������Cl2�������ӷ���ʽ���Ͳ���Cl2��ԭ��__________________________________________��
(5)NaClO2���ʿɷֽ�ΪNaClO3��NaCl��ȡ������NaClO2������һ�ݸ����ʵ�����ʹ֮���ʣ���һ���ϸ棬�������Һ�����ֱ�������FeSO4��Һ��Ӧʱ������Fe2�������ʵ���________(���ͬ��������ͬ�������жϡ�)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������������Ⱦ��Ϊ���أ����������������ü�����⡣
(1)���������һ�������ȼҵ��Ʒ������������������ķ������������£�
(��)����SO2�ķ���ͨ���ⱥ��ʳ��ˮ�����õ�����Һ�У���NaHSO3��Һ��
(��)����ⱥ��ʳ��ˮ�������巴Ӧ���Ƶ����ᡣ
(��)���������NaHSO3��Һ�У���Ӧ���õ���SO2������գ����ɵ�NaClѭ�����á�
��д������(��)��Ӧ�Ļ�ѧ����ʽ�� ��
��д������(��)�е�ⱥ��ʳ��ˮ�Ļ�ѧ����ʽ�� ��
��д������(��)��Ӧ�����ӷ���ʽ�� ��
(2)����ѧ���������Fe2����Fe3�������ӵĴ����ã������½�SO2������SO42-��ʵ��SO2�Ļ������á�ij�о���ѧϰС��ݴ���������·�������ʵ���������²ⶨת������SO2������SO42-��ת���ʡ�
�ٸ�С�������ͼװ����ʵ���Ҳⶨģ��������SO2�����������X��Һ������ ��(��д���)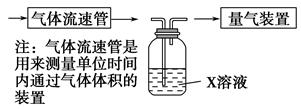
| A����ĵ�����Һ | B�����Ը��������Һ |
| C������������Һ | D���Ȼ�����Һ |
 ��ת���ʣ���֪�������٣�����ⶨ�������� �� ��
��ת���ʣ���֪�������٣�����ⶨ�������� �� ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��12�֣���ͼ��A��J�ֱ������ط�Ӧ�е�һ�����ʣ���֪A�ֽ�õ������ʵ�����B��C��D����֪B��DΪ�����µ���̬�����CΪ�����µ�Һ̬�����ͼ���в���������δ�����
����д���¿հף�
��1��A�Ļ�ѧʽΪ ��B�ĵ���ʽΪ ��
��2��д�����з�Ӧ�Ļ�ѧ����ʽ��
D+G��H ��
F+J��B+C+I ��
��3��0��3 mol I������C��Ӧת�Ƶ��ӵ����ʵ���Ϊ mol��
��4���ݻ�Ϊ10 mL���Թ��г���I��G�Ļ�����壬������ʢˮ��ˮ���У�ˮȫ�������Թܣ���ԭ���������I��G������ֱ�Ϊ mL�� mL��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���������ʵ�ת����ϵ�У�A��һ�ֹ��嵥�ʣ��ҳ����뵼����ϣ�E��һ�ְ�ɫ������F����������嵥�ʡ�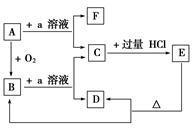
�ݴ���д��(1)B�Ļ�ѧʽ�� ��Ŀǰ���ִ�ͨѶ����B�ѱ����� ����Ҫԭ�ϡ�
(2)B��a��Һ��Ӧ�����ӷ���ʽ�� ��
(3)A��a��Һ��Ӧ�����ӷ���ʽ�� ��
(4)C�������ᷴӦ�����ӷ���ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������ˮ�к��ж������ӣ�ijУ��ѧ�о���ѧϰС���ͬѧΪ̽������ɣ���������ʵ�飬�ش�
��1������ˮʪ��ĺ�ֽ�е����������Ʊ�����ˮ���۲쵽 ����˵����
����ˮ�к��� ��
��2�����Թ��м��������Ŀ�״̼��ƣ��ټ�������������ˮ����ַ�Ӧ�����������ݲ�
���������ӷ���ʽ���ʹ����� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com