
��.ʵ����������500mL 0.2mol/L NaCl��Һ
��1����ʵ���õ�����������ҩ�ס��ձ�����Ͳ��������ƽ(���롢����)������������ͷ�ιܣ���ȱ�ٵIJ��������� ��
��2������ʱ�����ȡNaCl�������� g
��3�������ƹ����У�������������ȷ������£����в����ᵼ�������Ƶ���ҺŨ��ƫ�ߵ��� ����ѡ����ţ�
��û��ϴ���ձ��Ͳ�����
�ڼ�����ˮʱ�����������˿̶ȣ�ȡ������ˮʹҺ��ǡ�õ��̶���
������ƿ�����������������ˮ
�ܶ���ʱ���ӱ���
�ݶ���ʱ���ӱ���
��.��10�֣�ij����Ϊ���������һ��ѧ��ȤС����ʵ������Ըô�������Ԫ�صļ�̬����̽���������й����ϵ�֪Fe2+�ܱ�����KMnO4��Һ������ʹ����KMnO4��Һ��ɫ��HNO3����ǿ�����ԡ�����ɶ���Ԫ�ؼ�̬��̽����
��1������������衣
����1����������Ԫ��Ϊ+3�ۣ�
����2�� ��
����3����������Ԫ�ؼ���+3������+2��
��2�����ʵ�鷽����
��3�����ݣ�2����ʵ�鷽������ʵ�飺
����1��ȡһ������ϡ�������Թ��У�������������ӣ�Ŀ���� ��
����2��ȡ������Ʒ���Թ��У����봦���������Ტ���ȣ��õ�A��Һ��
����3��ȡA��Һ���Թܣ��μ�KSCN��Һ������������Ѫ��ɫ������� ������������Ѫ��ɫ�������1��3������
����4��Ϊ�˽�һ��ȷ������3���Ǽ���1���Ǽ���3��������ȡA��Һ���Թܣ�����KMnO4��Һ���������� ���������1��������֮�������3������
��4����˼
ijͬѧ������2�е�ϡ����ij�ϡ����õ���A��Һ����A��Һ�еμ�KSCN��Һ�����Ѫ��ɫ���ɴ˵ó��ô�������Ԫ��Ϊ+3�۵Ľ��ۡ����жϸý����Ƿ����
���������������������
��1��500 mL����ƿ��û��д����ƿ���ݻ�������ƿ��д�������÷֣���2�֣�
��2��5.9g��5.85�����÷֣���2�֣�
��3���ݣ�2�֣�
��1����������Ԫ��Ϊ+2�ۣ�2�֣�
��3����ȥ�������ܽ��������2�֣�����3��2��2�֣� ����4�����������Һ����ɫ��2�֣�
��4����������2�֣�
���������������1��������Һ����IJ�����������ƿ����ע������ƿ����ע�������500 mL����ƿ����2������500mL 0.2mol/L NaCl��Һ��������NaCl5.85 g������������ƽֻ�ܳ�����0.1g�����Ա���д��5.9g����3����ƫ�͢�ƫ�͢���Ӱ���ƫ�͢�ƫ��
��.��1�����������ļ���1��3��֪������2Ӧ���Ǵ�������Ԫ��Ϊ+2��
��3����������Ҳ���������ԣ��������������ӣ����Լ�����������ӵ�Ŀ���dz�ȥ�������ܽ��������
����3�������������ܺ�KSCN��Һ��Ӧ�Ժ�ɫ��������������Ѫ��ɫ�������2������������Ѫ��ɫ�������1��3������
����4���������Ӿ��л�ԭ�ԣ��ܱ����Ը��������Һ��������ɫ������������ָ��������Һ����ɫ���������1��������֮�������3������
��4������������������ԣ����������ὫFe2+������Fe3+���Ӷ�����ʵ����۲�ȷ���ʲ�������
���㣺������������������Ԫ�ػ��ϼ�̽����ʵ���ж�


 �п������п��Ծ����ϵ�д�
�п������п��Ծ����ϵ�д� ��������״Ԫ��ϵ�д�
��������״Ԫ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
Fe(OH)2�ܲ��ȶ���¶���ڿ��������ױ�������������Ӧ�ķ���ʽΪ��
Ϊ�˻�ð�ɫ��Fe(OH)2�����������ò���Fe3+��FeSO4��Һ�벻��O2������ˮ���Ƶ�NaOH��Һ��Ӧ�Ʊ���
��1����FeSO4������������FeSO4��Һʱ�Ҿ��û������ ����ֹFe2+��������
��2����ȥ����ˮ���ܽ��O2������ �ķ�����
��3������Fe3+������Լ��� ����������鷽���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����X����������������ͭ��ɣ�ȡ������ȵ�������������ͼ����ʵ�飺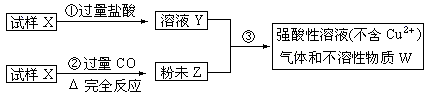
��1����д�����ۢ��з�����ȫ����Ӧ�����ӷ���ʽ��____________________________________��
��2��Ҫʹ����Xת��Ϊ��ĩZ������CO�⣬������ʹ�� ��
| A������ | B����̿ | C������ | D������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����������������������������ֽ������䵥�ʺͻ����ﱻ�㷺Ӧ�á�
��1����������Һ�����ڽ�����ͭ������Ҫ��ӦΪ��
CuFeS2��4Fe3��=Cu2����5Fe2����2S��CuFeS2��SΪ��2�ۣ�FeΪ��2�ۣ���
���ڸ÷�Ӧ������˵���У���ȷ���� ��ѡ����ţ���
a�������ʷ���ĽǶȿ���CuFeS2���ںϽ� b����Ӧ������ֻ��һ��Ԫ�ر���ԭ
c��CuFeS2����������������ԭ�� d����ת��1 mol����ʱ������16 g S
��2��������أ�K2FeO4����һ������ˮ����������ˮ�з�����Ӧ����Fe��OH��3���塣�÷�Ӧ�У�������ر��� ��������ԡ���ԭ�ԡ�����Fe��OH��3������о�ˮ���ã���ԭ���� ��
��3��ijͬѧȡһ���������������һЩС�ף�Ȼ��ȡһҩ�������Ʒ�ĩ�����������ã�������ͼ��ʾװ���ڵ��۵�©���У�ͼ������̨������û�л�������
��˳��д���ù����з�����Ӧ�����ӷ���ʽ��
�� ��
�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ʯ����ȡ�طʺ�������������Ҫԭ�ϣ�����ʯ����ɺ��������ƣ�����������������������������ʡ�����ʵ�鲽������ͼ��ʾ��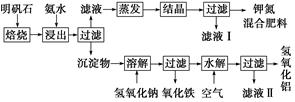
��������ͼʾ�����������գ�
��1������ʯ���պ���ϡ��ˮ����������500 mLϡ��ˮ(ÿ������39.20 g��)��ҪȡŨ��ˮ(ÿ������251.28 g��)__________mL���ù��Ϊ__________mL��Ͳ��ȡ��
��2����ˮ������õ���������ϵ�����ˣ���Һ�г�K����SO42-�⣬���д�����NH4+������NH4+�ķ�����_________________________________________________________________��
��3��д�����������������ʵĻ�ѧʽ___________________________________��
��4����Һ��ijɷ���ˮ��______________________��
��5��Ϊ�ⶨ��Ϸ���K2SO4��(NH4)2SO4�мصĺ��������������в��裺
�ٳ�ȡ�ص�������������ˮ����������__________��Һ��������ɫ������
��__________��__________��__________(������дʵ���������)��
����ȴ�����ء�
��6��������Ϊm g�����������ʵ���Ϊn mol����������K2SO4�����ʵ���Ϊ__________mol(�ú���m��n�Ĵ���ʽ��ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���ִ���ҵ�����Ȼ���Ϊԭ���Ʊ�������ֹ����������£�
��֪NaHCO3�ڵ������ܽ�Ƚ�С��
��Ӧ��NaCl+CO2+NH3+H2O NaHCO3��+NH4Cl������ĸҺ�����ַ�����
NaHCO3��+NH4Cl������ĸҺ�����ַ�����
��1����ĸҺ�м���ʯ���飬�ɽ�����________ѭ�����á�
��2����ĸҺ��ͨ��NH3������ϸС��ʳ�ο��������£��ɵõ�NH4Cl���塣��д��ͨ��NH3���ܽ�Ƚ�С����ʽ̼����ת��Ϊ�ܽ�Ƚϴ��̼���ε����ӷ���ʽ ___________��
��ij��ѧС��ģ�⡰�����Ƽ������NaCl��NH3��CO2��ˮ��Ϊԭ���Լ���ͼ��ʾװ����ȡNaHCO3��Ȼ���ٽ�NaHCO3�Ƴ�Na2CO3��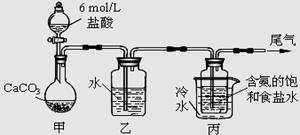
��1��װ�ñ�����ˮ�������� ��
��2����װ�ñ��в�����NaHCO3��ȡNa2CO3ʱ����Ҫ���е�ʵ�������_______��ϴ�ӡ����ա�NaHCO3ת��ΪNa2CO3�Ļ�ѧ����ʽΪ ��
��3�����ڣ�2�������յ�ʱ��϶̣�NaHCO3���ֽⲻ��ȫ����С���һ�ݼ�����t1 min��NaHCO3 ��Ʒ����ɽ���������̽����
ȡ������t1 min��NaHCO3��Ʒ29��6 g��ȫ����ˮ�Ƴ���Һ��Ȼ�������Һ�л����صμ�ϡ���ᣬ�����Ͻ��衣��������ļ��룬��Һ���й����ӵ����ʵ����ı仯��ͼ��ʾ��
������a��Ӧ����Һ�е�������___________�������ӷ�����ͬ��������c��Ӧ����Һ�е�������___________������Ʒ��NaHCO3��Na2CO3�����ʵ���֮���� �� 21
��4����ȡ21��0 g NaHCO3���壬������t2 rnin��ʣ����������Ϊl4��8 g������Ѵ�ʣ�����ȫ�����뵽200 mL 2 mol?L��1�������У����ַ�Ӧ����Һ��H+ �����ʵ���Ũ��Ϊ____________������Һ����仯���Բ��ƣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������֮�������·�Ӧ��ϵ��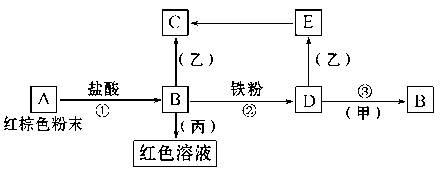
��֪��Eת����C�������ǣ���ɫ����Ѹ�ٱ�Ϊ����ɫ,����Ϊ���ɫ���ش�
��1��д���������ʵĻ�ѧʽ����________,��________��
��2��д��E��C��Ӧ�Ļ�ѧ����ʽ��___________________________��Ϊ�˻��E�������������Ƶ�D��Һ���ò���O2������ˮ���Ƶ�����Һ��Ӧ�Ʊ���
��3������Ӧ������������D��Һʱ����û������ ��
��4��д��Fe��H20��һ�������·�Ӧ�Ļ�ѧ����ʽ ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(1)���û�ѧ��Ӧ�����Ʊ��������ʡ�ʵ������ͭ�Ʊ�NO2�����ӷ���ʽΪ_____________ ______��
��2����ҵ�ϣ���ͭ����Ҫ�ɷ�CuFeS2������ȡͭ����Ҫԭ�ϣ��ɲ��û�������������ͭ���ù��յ��м���̻ᷢ����Ӧ��Cu2S��2Cu2O===6Cu+SO2�����÷�Ӧ�л�ԭ��Ϊ_______ (�ѧʽ)��ÿ����1mol Cu����Ӧ��ת�Ƶ��ӵ����ʵ���Ϊ___________��
��ͭ��ұ��ͭ������¯������Fe2O3��FeO��SiO2��Al2O3�����Ʊ�Fe2O3������Ϊ��
����ϡ�����ȡ¯�������ˡ� ����Һ���������ټ������NaOH��Һ�����ˣ�������ϴ�ӡ�������յ�Fe2O3�� ��������Ϣ�ش��������⣺
a��ͨ�������ڣ�¯���е�Al2O3����� ��д���ӣ���
b��ѡ���ṩ���Լ������ʵ����֤¯���к���FeO��
�ṩ���Լ���ϡ���� ϡ���� KSCN��Һ ����KMnO4��Һ NaOH��Һ ��ˮ
��ѡ�Լ�Ϊ ��
֤��¯���к���FeO��ʵ������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����������������õķ���ʴ�ԣ��ڹ�����ҵ���зdz���Ҫ�����ã��������ȷ�Ӧ��ɸֹ�ĺ��ӷdz�����Ѹ�١����������գ�
��1��������©���н����ȼ���Ͼ��Ⱥ��������ȷ�Ӧ�IJ����ǣ� ��
��2��������ͬ���ڡ��ؿ���衢���ĺ����� �����,����=����SiO2�ǹ����β�����Na2CaSi6O14����Ҫ�ɷ֣�Na2CaSi6O14Ҳ��д��Na2O��CaO��6SiO2���Ƴ�ʯ��NaAlSi3O8������������ʽ ����ʯ���������Σ���ͬ�ʯ����ԭ�ӵ����ʵ���������ͬ���ɴ˿���֪�Ƴ�ʯ�Ļ�ѧʽΪ ��
��3��ij���Ͻ���Al��Si��Cu��Mg��ɡ��ٳ�ȡ100g�����Ͻ���Ʒ���ֳɵ�������A��B���ݡ���A�ݼ�������NaOH��Һ��B�ݼ���������ϡ���ᡣ�ڴ����ݷ�Ӧ�ﶼ��ַ�Ӧ֮�Ƶ������������1.60g���ռ��õ������������������2240mL(��״����)������Ʒ��Si��Mg�����ʵ����ֱ��� �� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com