
Fe(OH)2�ܲ��ȶ���¶���ڿ��������ױ�������������Ӧ�ķ���ʽΪ��
Ϊ�˻�ð�ɫ��Fe(OH)2�����������ò���Fe3+��FeSO4��Һ�벻��O2������ˮ���Ƶ�NaOH��Һ��Ӧ�Ʊ���
��1����FeSO4������������FeSO4��Һʱ�Ҿ��û������ ����ֹFe2+��������
��2����ȥ����ˮ���ܽ��O2������ �ķ�����
��3������Fe3+������Լ��� ����������鷽���� ��
4Fe(OH)2+O2+2H2O=4Fe(OH)3
��1������ ��2����� ��3��KSCN��Һ��ȡ����Һ�������Թ��У�����KSCN��Һ����Һ��죬��ԭ��Һ�к���Fe3+������������Ҳ�У�
�������������Fe(OH)2�ܲ��ȶ���¶���ڿ�����Ѹ���а�ɫ��ɻ���ɫ�����ձ�ɺ��ɫ�����̼��𰸣�Fe2+���ȶ������бȽ�ǿ�Ļ�ԭ�ԣ����ױ�����ΪFe3+�����ڱ���FeSO4��Һʱ�������۷�ֹ�䱻�������¶�Խ�ߣ�������ܽ��ԽС��������к������ˮ�����������ϵͣ�Fe3+�ļ��鳣��KSCN��Һ����Һ��ΪѪ��ɫ��
���㣺������Ԫ�صĻ��������ʼ�����


 ȫ�ܲ��һ���þ�ϵ�д�
ȫ�ܲ��һ���þ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ͼ��ʾ��װ�ý���ʵ�飬�ش��������⣺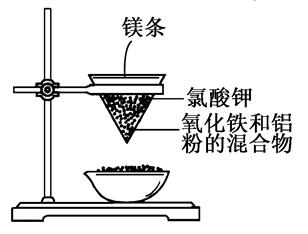
��1��д���÷�Ӧ�Ļ�ѧ����ʽ��_____________________��
�ڸ÷�Ӧ��________����������________�ǻ�ԭ�����÷�Ӧ��Ϊ________��Ӧ��
��2��ѡ����ʵ���ʵ�����������д�ں����ϣ���____________��
��þ������ȼ�գ��ڷų��������ȣ�������ҫ�۵Ĺ�â���������䣻��ֽ©�����²����մ������к���״̬��Һ�������������ڵ�ϸɳ�ϣ�Һ����ȴ���Ϊ��ɫ���塣
��3��д�����в��������ʵ����ã��ڲ�ֽ©���ײ���һ���ף�________________��������ʢɳ��______________��þ���������______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����������Ҫ�ɷ���Al2O3���������Ĺ�������ʾ��ͼ���£�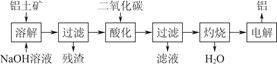
��1��������ɵ���������Һ�� ����ϲ㡱���²㡱�������ʱ�������ĵĵ缫�� ���������������������
��2��д��ͨ�����������̼�ữʱ��Ӧ�����ӷ���ʽ
��
��3������Ʊ���ʱ����������ʯ��Na3AlF6������������ ����ҵ�Ͽ����÷��������塢���������ʹ����ڸ��������·�����Ӧ����ȡ����ʯ��д���÷�Ӧ�Ļ�ѧ����ʽ ��
��4������������������������������Fe��Si�����ʣ����õ�ⷽ����һ���ᴿ���õ��ص����������� ���ѧʽ���������ĵ缫��ӦʽΪ ��
��5���Խ�����Ʒ���п���ʴ���������ӳ���ʹ��������
�ٿ���һ���������е�⣨��ͼ������ʱ��������γ��������������Ĥ����缫��ӦʽΪ ��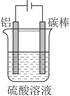
�ڸֲĶ������ܷ�ֹ�ֲĸ�ʴ����ԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ijͬѧʵ��̽�������Ļ�ԭ�Բ�����ϵ��ʵ�顣
��1�����������Ϻ�ɫͭ˿�Ƽ�Ȧ���ھƾ��ƻ����ϼ��ȣ���ͭ˿��ں��Ƚ�ͭ˿�������ɵ��Ȼ�茶�������̷����а�ɫ�������ɣ��ó�ͭ˿��ͭ˿��ת��Ϊ�������Ϻ�ɫ���ں���ͭ��ʱ���Ȼ�麟�ȥͭ�����������ͭ�������˸�ԭ��������ɸ÷�Ӧ��
��2����������װ�ã����������������MxOy��Ӧ����M��H2��H2O��ͨ����������ˮ���������ⶨM�����ԭ��������a���Լ���Ũ��ˮ��
������a������Ϊ__________������b��װ�˵��Լ�������____________.
�ڰ�����������ȷ��װ������˳��Ϊ������ţ�װ�ÿ��ظ�ʹ�ã���___________��
��ʵ�����ʱ��Ӧ����__________������ţ���
I��Ϩ��Aװ�õľƾ���
II��ֹͣ��a�еμ�Һ��
����ʵ����ȷ��ȡ���������������Ϊmg����ȫ��Ӧ�������ˮ������Ϊng����M�����ԭ������Ϊ__________(�ú�X��y��m��n��ʽ�ӱ�ʾ)��
��3��������������MxOyΪFe2O3������Ӧ���������ϡ���ᣬȻ��μ�KSCN��Һû�������Ա仯���Ʋ�ù���ɷֿ����ǣ�����ѧʽ�����±�������Ϊ�м��ֿ�����֣���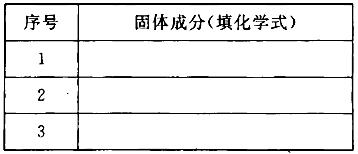
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����������壨FeSO4��7H2O����ҽҩ������Ѫ����ij����С���ͬѧ���ⶨ�ò�Ѫ������Ԫ�صĺ�����ʵ�鲽�����£�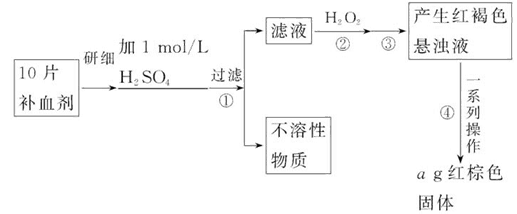
��ش��������⣺
��1��֤���������Һ�к���Fe2+�ķ����ǣ�ȡ�����ȵμ�KSCN��Һ���ٵμ�_______���ù��̵�����Ϊ��__________________________________________��
��2������ڼ������H2O2��Ŀ���ǣ�____________________________________��
��3��������з�Ӧ�����ӷ���ʽ�ǣ�_____________________________________��
��4���������һϵ�д����IJ������裺���ˡ�_______�����ա�_______��������
��5����ʵ����������ģ���ÿƬ��Ѫ���к���Ԫ�ص�����Ϊ_______g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij����С���һЩ�������ʺͻ���������ʽ���̽����
(1)�±�Ϊ�������Ȼ�ͭ��Һ��Ӧ��ʵ�鱨���һ���֣�
| ʵ�鲽�� | ʵ������ |
| ����ĥ������Ƭ(����)����һ��Ũ�ȵ�CuCl2��Һ�� | �������ݣ��������ɵĺ�ɫ���壬��Һ��Ϊ��ɫ |
| ��Ӧ������������Һ���� | |
| ��ɫ����������ˮϴ�Ӻ����ڳ�ʪ������ | һ��ʱ�������ɺ�ɫ��Ϊ��ɫ[������Ҫ�ɷ�ΪCu2(OH)2CO3] |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪A��K��Ϊ��ѧ��ѧ�еij������ʣ�����֮���ת����ϵ��ͼ��ʾ������A��DΪ�������ʣ���Ӧ���������ɵ�ˮ���������ֲ�������ȥ��
��ش��������⣺
��1��A��B��Ӧ�Ļ�ѧ����ʽ�ǣ�______________________________________��
��2����F��ͨ������CO2����K�����ӷ���ʽΪ��_________________________��
��3��A��NaOH��Һ��Ӧ�����ӷ���ʽ�ǣ�_________________________________________________��
��4���ټ�������J����Һ�н������ӵķ����ǣ�_______________________________________________��
�ڽ�����E����ˮ������Һ�����ԣ�ԭ���ǣ������ӷ���ʽ��ʾ��____________��
��ij��Ч��ˮ������D��OH��SO4�ۺϵõ��ġ���ҵ����DSO4��ϡ�������������Ϊԭ�����Ʊ�D��OH��SO4����Ӧ����NO���ɣ��÷�Ӧ�Ļ�ѧ����ʽ�ǣ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ӡˢ��·���������Ϻ�ͭ�����϶��ɣ�������ӡˢ��·ʱҪ����Һ��Ϊ����ʴҺ��
�ܽ�ͭ��
��1��д���÷�Ӧ�Ļ�ѧ����ʽ��________________________________________;
��2��д��FeCl3��������Zn��Ӧ�Ļ�ѧ����ʽ��__________________________________;
��3����ʴҺ�þû�ʧЧ�������Ի������ã�
����Ҫ�õ�����ͭ�������Լ���ʵ�ֵ��ǣ�����ţ�
A������ B���� C������ D��ϡ���� E����
����Ҫ��ת��Ϊ�������Լ���ʵ�ֵ��ǣ�����ţ�
A������ B���� C������ D��ϡ���� E����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��.ʵ����������500mL 0.2mol/L NaCl��Һ
��1����ʵ���õ�����������ҩ�ס��ձ�����Ͳ��������ƽ(���롢����)������������ͷ�ιܣ���ȱ�ٵIJ��������� ��
��2������ʱ�����ȡNaCl�������� g
��3�������ƹ����У�������������ȷ������£����в����ᵼ�������Ƶ���ҺŨ��ƫ�ߵ��� ����ѡ����ţ�
��û��ϴ���ձ��Ͳ�����
�ڼ�����ˮʱ�����������˿̶ȣ�ȡ������ˮʹҺ��ǡ�õ��̶���
������ƿ�����������������ˮ
�ܶ���ʱ���ӱ���
�ݶ���ʱ���ӱ���
��.��10�֣�ij����Ϊ���������һ��ѧ��ȤС����ʵ������Ըô�������Ԫ�صļ�̬����̽���������й����ϵ�֪Fe2+�ܱ�����KMnO4��Һ������ʹ����KMnO4��Һ��ɫ��HNO3����ǿ�����ԡ�����ɶ���Ԫ�ؼ�̬��̽����
��1������������衣
����1����������Ԫ��Ϊ+3�ۣ�
����2�� ��
����3����������Ԫ�ؼ���+3������+2��
��2�����ʵ�鷽����
��3�����ݣ�2����ʵ�鷽������ʵ�飺
����1��ȡһ������ϡ�������Թ��У�������������ӣ�Ŀ���� ��
����2��ȡ������Ʒ���Թ��У����봦���������Ტ���ȣ��õ�A��Һ��
����3��ȡA��Һ���Թܣ��μ�KSCN��Һ������������Ѫ��ɫ������� ������������Ѫ��ɫ�������1��3������
����4��Ϊ�˽�һ��ȷ������3���Ǽ���1���Ǽ���3��������ȡA��Һ���Թܣ�����KMnO4��Һ���������� ���������1��������֮�������3������
��4����˼
ijͬѧ������2�е�ϡ����ij�ϡ����õ���A��Һ����A��Һ�еμ�KSCN��Һ�����Ѫ��ɫ���ɴ˵ó��ô�������Ԫ��Ϊ+3�۵Ľ��ۡ����жϸý����Ƿ����
���������������������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com