
��12�֣���֪��A��B��C��D���ֶ�����Ԫ�أ�A��D��ԭ������֮�͵���B��C��ԭ������֮�ͣ���DԪ����ɵĵ�����ͨ��״���³ʻ���ɫ��B��C��D����Ԫ��λ��ͬһ���ڣ�A��B��C����Ԫ�ص�����������Ӧ��ˮ����ֱ�ΪX��Y��Z���Ҵ�������ת����ϵ�����ƶϻش��������⡣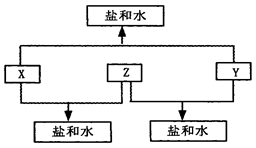
��1��DԪ��ԭ�ӵĽṹʾ��ͼΪ ��
��2��A��B��C����Ԫ�ص�ԭ�Ӱ뾶��С�����˳��Ϊ ����Ԫ�ط��ű�ʾ����
��3��Y��CԪ�ص������������Է�����Ӧ���÷�Ӧ�����ӷ���ʽΪ ��
��4��A��D��Ԫ�ص���̬�⻯��֮����Է�Ӧ����һ���Σ����ε�ˮ��Һ�� ����ᡱ��������С����ԣ���ˮ��Һ�и�����Ũ����С�����˳��Ϊ ���þ������ӷ��ű�ʾ����
��5��ʵ�����У�Ӧ��X��Ũ��Һ��������ɫ�Լ�ƿ�У���ԭ���� ���û�ѧ����ʽ��ʾ����
��12�֣�
��1�� ��2�֣� ��2��N<Al<Na��2�֣�
��2�֣� ��2��N<Al<Na��2�֣�
��3��Al2O3+2OH-+3H2O=2[Al(OH)4]- ��2�֣�
��4���ᣨ2�֣�  ��2�֣�
��2�֣�
��5�� ��2�֣�
��2�֣�
���������������Ϊ��DԪ����ɵĵ�����ͨ��״���³ʻ���ɫ������D����Ԫ�ء�B��C��D����Ԫ��λ��ͬһ���ڣ��ڷ�Ӧ��X��Y��Z���������κ�ˮ����������һ�ֿ϶��ȿ������ᷴӦҲ������Ӧ�����Կ϶���һ��������������A�ڵڶ����ڿ���������������Ӧ����A�ǵ�Ԫ�أ�����A��D��ԭ������֮�͵���B��C��ԭ������֮�ͣ���B��CӦ�����ơ�����
��1����ԭ�ӵĽṹʾ��ͼ��
��2���������ڱ���ͬ����ԭ�Ӱ뾶Խ��ԽС��ͬ����뾶Խ��Խ����N<Al<Na��
��3����Ϊ�����������������������������κ�ˮ��Al2O3+2OH-=AlO2��+H2O��Al2O3+2OH-+3H2O=2[Al(OH)4]-��
��4���Ȼ����ǿ�������Σ������ԣ�笠�����Ҫˮ�⣬������ ��
��
��5��Ũ����������ֽ⣬����ʽ��
���㣺������Ԫ�ص��ƶϣ�ԭ�ӽṹ�����������������������ԣ��ε�ˮ�⼰Ũ��������ʡ�
�����������漰֪ʶ��϶࣬����ͻ�ƿڼ�����˼·����ץס�������ѶȲ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2011?�����ģ����ѧ--ѡ�����ʽṹ������
��2011?�����ģ����ѧ--ѡ�����ʽṹ�������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��֪��A��B��C��D��E��F��XΪ���ڱ���ǰ�����ڵ�����Ԫ�أ����ǵ�ԭ��������������A�����ڱ���ԭ�Ӱ뾶��С��Ԫ�أ�B�Ļ�̬ԭ����3����ͬ���ܼ������ܼ��е�������ȣ�D�Ļ�̬ԭ��2p�ܼ��ϵ�δ�ɶԵ�������Bԭ�ӵ���ͬ��D2-������E2+���Ӿ�����ͬ���ȶ����Ӳ�ṹ��F�С����������֮�ƣ�F4+���Ӻ��ԭ�ӵĺ�������Ų���ͬ��X�Ļ�̬ԭ�ӵļ۵����Ų�ʽΪ3d84s2��
��֪��A��B��C��D��E��F��XΪ���ڱ���ǰ�����ڵ�����Ԫ�أ����ǵ�ԭ��������������A�����ڱ���ԭ�Ӱ뾶��С��Ԫ�أ�B�Ļ�̬ԭ����3����ͬ���ܼ������ܼ��е�������ȣ�D�Ļ�̬ԭ��2p�ܼ��ϵ�δ�ɶԵ�������Bԭ�ӵ���ͬ��D2-������E2+���Ӿ�����ͬ���ȶ����Ӳ�ṹ��F�С����������֮�ƣ�F4+���Ӻ��ԭ�ӵĺ�������Ų���ͬ��X�Ļ�̬ԭ�ӵļ۵����Ų�ʽΪ3d84s2��

| 3 |
| ||
| 3 |
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��֪����A��B��C��D�����µķ�Ӧ��ϵ������A ��B��ȼ��ʱ������ʲ�ɫ�� C��B��ȼ��ʱ�����ػ�ɫ���̣�E��ˮ��Һ������ɫ��G��һ�ֺ�ɫ���壮
��֪����A��B��C��D�����µķ�Ӧ��ϵ������A ��B��ȼ��ʱ������ʲ�ɫ�� C��B��ȼ��ʱ�����ػ�ɫ���̣�E��ˮ��Һ������ɫ��G��һ�ֺ�ɫ���壮
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com