


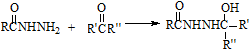 �������
����д���� �ͼ״�Ϊ��Ҫԭ���Ʊ�
�ͼ״�Ϊ��Ҫԭ���Ʊ� �ĺϳ�·������ͼ�����Լ����ã����ϳ�·������ͼʾ�����£�
�ĺϳ�·������ͼ�����Լ����ã����ϳ�·������ͼʾ�����£�| Ũ���� |
| 170�� |
| Br2 |
 ��Ӧ��ͬ���칹��Ϊ-Cl��-COOH��λ���칹��
��Ӧ��ͬ���칹��Ϊ-Cl��-COOH��λ���칹�� ��������
�������� ����״���Ӧ����
����״���Ӧ���� ������H2NNH2��Ӧ����
������H2NNH2��Ӧ���� ����
���� ��Ӧ����
��Ӧ���� �������Ũ���������¿�����Ŀ���
�������Ũ���������¿�����Ŀ��� ��
�� ��
�� ��Ӧ��ͬ���칹��Ϊ-Cl��-COOH��λ���칹����ĿΪC52=10���ʴ�Ϊ��10��
��Ӧ��ͬ���칹��Ϊ-Cl��-COOH��λ���칹����ĿΪC52=10���ʴ�Ϊ��10�� ��������
�������� ����״���Ӧ����
����״���Ӧ���� ������H2NNH2��Ӧ����
������H2NNH2��Ӧ���� ����
���� ��Ӧ����
��Ӧ���� �������Ũ���������¿�����Ŀ����ʺϳ�·�߹�������ͼΪ��
�������Ũ���������¿�����Ŀ����ʺϳ�·�߹�������ͼΪ�� ��
�� ��
��

 ������״Ԫ���Ծ�ϵ�д�
������״Ԫ���Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| Ũ���� |
| �� |
| Ũ���� |
| �� |






�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

 ��
��






 ������ͬ�ĺ�����Ԫ�ӻ������ŵ�ͬ���칹�干��
������ͬ�ĺ�����Ԫ�ӻ������ŵ�ͬ���칹�干�� ��
�� Ϊԭ�ϣ��ϳ�
Ϊԭ�ϣ��ϳ� ���ϳɹ��������Լ���ѡ���ϳ�·������ͼʾ�����£�
���ϳɹ��������Լ���ѡ���ϳ�·������ͼʾ�����£�| Ũ���� | 170�� |
 BrH2C-CH2Br��
BrH2C-CH2Br��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꽭��ʡ�����и�����ǰģ�⻯ѧ�Ծ��������棩 ���ͣ��ƶ���
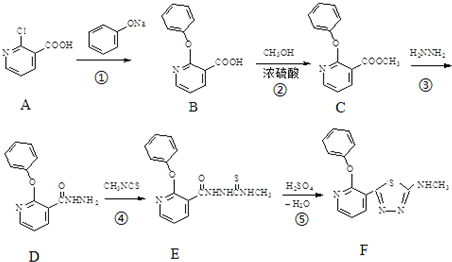
ij��ɫӫ�����F�ĺϳ�·�����£�

��1��������B�к��еĺ��������ŵ������� ��
��2��д��������CH3NCS�Ľṹʽ�� ��
��3���ϳ�·�������ڼӳɷ�Ӧ�ķ�Ӧ�� ��������ţ�
��4����Ӧ����һ����Ϊ�״����仯ѧ����ʽΪ ��
��5����A������ͬ�ĺ�����Ԫ�������ŵ�ͬ���칹�干�� �֣�������������֪������Ԫ���뱽���ṹ���ƣ���
��6����֪�� ����д����
����д���� �ͼ״�Ϊ��Ҫԭ���Ʊ�
�ͼ״�Ϊ��Ҫԭ���Ʊ� �ĺϳ�·������ͼ�����Լ����ã����ϳ�·������ͼʾ�����£�
�ĺϳ�·������ͼ�����Լ����ã����ϳ�·������ͼʾ�����£�
CH3CH2OH  CH2��CH2
CH2��CH2 BrCH2��CH2Br
BrCH2��CH2Br
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��0110 ģ���� ���ͣ������


 ������ͬ�ĺ�����Ԫ�ӻ������ŵ�ͬ���칹�干��_________ �֣�������������֪������Ԫ�ӻ��뱽���ṹ���ƣ���
������ͬ�ĺ�����Ԫ�ӻ������ŵ�ͬ���칹�干��_________ �֣�������������֪������Ԫ�ӻ��뱽���ṹ���ƣ��� ��
�� Ϊԭ�ϣ��ϳ�
Ϊԭ�ϣ��ϳ� ���ϳɹ��������Լ���ѡ���ϳ�·������ͼʾ�����£�
���ϳɹ��������Լ���ѡ���ϳ�·������ͼʾ�����£� 
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com