�л���ѧ֪ʶ��������Ӧ�ù㷺��
��1�����ࡢ��֬�͵������Ƕ����Ժ�ֲ����ʳ���еĻ���Ӫ�����ʣ�
�������й�˵���У���ȷ����
BCEF
BCEF
��
A���ޡ��顢ľ�ġ���˿����Ҫ�ɷֶ�����ά��
B����֬�Dz���������ߵ�Ӫ������
C���������������ڷ���ˮ���������ɰ�����
D����������������
E�����ۡ���ά�ء������ʶ�����Ȼ�߷��ӻ�����
F������炙�����Ǧ��Һ���뵽��������Һ�У������ʶ��ܴ���Һ������
��������������Ҫ����ĵ��ǣ�������һ��Ӫ�����ʣ����������ƾ��ȹ�ҵ�ϣ�д�������Ƿ���������Ӧ�Ļ�ѧ����ʽ��
CH
2OH��CHOH��
4CHO+2Ag��NH
3��
2OH
2Ag��+CH
2OH��CHOH��
4COONH
4+3NH
3+H
2O
CH
2OH��CHOH��
4CHO+2Ag��NH
3��
2OH
2Ag��+CH
2OH��CHOH��
4COONH
4+3NH
3+H
2O
��
��2��ƻ���᳣������ˮ���ǹ������Ӽ�����ṹ��ʽΪ
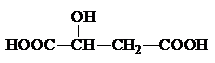
���÷����й����ŵ�����Ϊ
�Ȼ����ǻ�
�Ȼ����ǻ�
�����Ժʹ������ʷ���
����
����
��Ӧ�������Է�����������ˮ���������ᣬ��������ʹ��ˮ��ɫ����������Ľṹ��ʽΪ
HOOC-CH=CH-COOH
HOOC-CH=CH-COOH
��
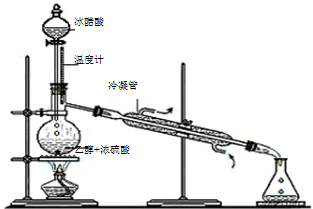
��3��ʵ���Һϳ����������IJ������£���Բ����ƿ�ڼ����Ҵ���Ũ��������ᣬƿ����ֱ��װͨ����ȴˮ�������ܣ�ʹ��Ӧ��������������ΪҺ��������ƿ�ڣ������Ȼ���һ��ʱ�������װ�ý���������ͼ1��ʾ�����õ������Ҵ��������ˮ�����������ֲ�Ʒ����ش��������⣺����֪���Ҵ������ᡢ���������ķе�������78.4�桢118�桢77.1�棩
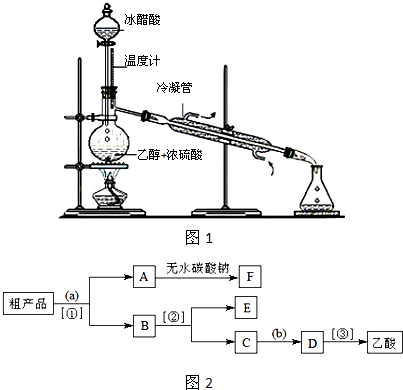
������ƿ�г��˼����Ҵ���Ũ����������⣬��Ӧ���뼸�����Ƭ����Ŀ����
��ֹ��ƿ�е�Һ�屩��
��ֹ��ƿ�е�Һ�屩��
��
���������������ķ�Ӧ�ǿ��淴Ӧ����Ӧ�ﲻ����ȫ����������Ӧһ��ʱ��ʹﵽ�˸÷�Ӧ���ȣ����ﵽ��ѧƽ��״̬������������˵���÷�Ӧ�Ѵﵽ��ѧƽ��״̬���У�����ţ�
BDE
BDE
��
A����λʱ�������1mol����������ͬʱ����1molˮ
B����λʱ�������1mol����������ͬʱ����1mol����
C����λʱ�������1mol�Ҵ���ͬʱ����1mol����
D������Ӧ���������淴Ӧ���������
E��������и����ʵ�Ũ�Ȳ��ٱ仯
��������뺬���ᡢ�Ҵ���ˮ�����������ֲ�Ʒ����ͼ2��ʾ�Ƿ��������������ͼ��
�Լ�a��
����Na2CO3��Һ
����Na2CO3��Һ
�����뷽������
��Һ
��Һ
�����뷽������
����
����
���Լ�b��
��Ũ������
��Ũ������
��
��д��C��D ��Ӧ�Ļ�ѧ����ʽ
2CH3COONa+H2SO4��2CH3COOH+Na2SO4
2CH3COONa+H2SO4��2CH3COOH+Na2SO4
��

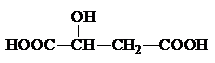 ���÷����й����ŵ�����Ϊ
���÷����й����ŵ�����Ϊ



 �л���ѧ֪ʶ��������Ӧ�ù㷺��
�л���ѧ֪ʶ��������Ӧ�ù㷺��