
|
��25�桡101 kPa�£�����(��ѧʽΪP4)������(��ѧʽΪP)ȼ�յ��Ȼ�ѧ����ʽ�ֱ�Ϊ��P4(s)��5O2(g)��P4O10(s)����H����3093.2 kJ/mol 4P(s)��5O2(g)��P4O10(s)����H����2954.0 kJ/mol �ɴ��жϣ�����˵����ȷ���� | |
| [����] | |
A�� |
�ɺ���ת��Ϊ���������ȷ�Ӧ��������ʱ���������Ȱ��� |
B�� |
�ɺ���ת��Ϊ�����Ƿ��ȷ�Ӧ��������ʱ���������Ȱ��� |
C�� |
�ɺ���ת��Ϊ�����Ƿ��ȷ�Ӧ��������ʱ���������Ȱ��� |
D�� |
�ɺ���ת��Ϊ���������ȷ�Ӧ��������ʱ���������Ȱ��� |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��������ʦ����2011��2012ѧ��߶���ѧ�����п��Ի�ѧ���� ���ͣ�022
ú�������Ǹ�Ч����������ú̿����Ҫ;��֮һ��
(1)��25�桢101 kPaʱ��H2��O2��������1 mol��H2O(g)�ų�241.8 kJ�����������Ȼ�ѧ����ʽΪ________��
��֪����C(s)��O2(g)��CO2(g)������H����393.5 kJ/mol
��CO(g)��1/2��O2(g)��CO2(g)������H����283.0 kJ/mol
��̿��ˮ������Ӧ�ǽ�����ú��Ϊ����ȼ�ϵķ�����C(s)��H2O(g)��CO(g)��H2(g)����H��________kJ/mol��
(2)CO������H2O(g)��һ��������Ӧ��CO(g)��H2O(g)![]() CO2(g)��H2(g)����H��0�ڼס��ҡ������������ܱ������У���ʼʱ�����±�����Ͷ�ϣ���800��ʱ�ﵽƽ��״̬��K��1.0��
CO2(g)��H2(g)����H��0�ڼס��ҡ������������ܱ������У���ʼʱ�����±�����Ͷ�ϣ���800��ʱ�ﵽƽ��״̬��K��1.0��
�ٸ÷�Ӧ��ƽ�ⳣ������ʽΪ________��
��ƽ��ʱ����������CO��ת������________��
������CO��ת���ʣ���________�ף���________�ף�(�����������������)
�۱������У�ͨ���ı��¶ȣ�ʹCO��ƽ��ת�������ߣ����¶�________(����ߡ����͡�)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ������ѧ2012�����3��˫����ϰ(һ)��ѧ���� ���ͣ�013
|
NAΪ�����ӵ����������и�����������ȷ���� ��0.2 mol��H2O2��ȫ�ֽ�ת�Ƶĵ�����Ϊ0.4NA ��25�桢101 k��Pa�£�16 g��O3��O2��������к��е���ԭ����ΪNA ��0.1 mol��FeCl3�����ˮ�γɵĽ������ӵ���ĿΪ0.1NA ��1 mol��N2��3 mol��H2��һ�������µ��ܱ������г�ַ�Ӧ�������ڵķ���������2NA | |
| [����] | |
A�� |
�٢ڢ� |
B�� |
�٢ڢ� |
C�� |
�٢ڢۢ� |
D�� |
�ڢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�������к�����2011�������ģ���ۻ�ѧ���� ���ͣ�022
ú�������Ǹ�Ч����������ú̿����Ҫ;��֮һ��
(1)
��25����101 kPaʱ��H2��O2��������1 mol��H2O(g)�ų�241.8 kJ�����������Ȼ�ѧ����ʽΪ________����֪����C(s)��O2(g) CO2(g)����H����393.5 kJ/mol
CO2(g)����H����393.5 kJ/mol
��CO(g)��![]() O2(g)
O2(g) CO2(g)����H����283
CO2(g)����H����283
��̿��ˮ������Ӧ�ǽ�����ú��Ϊ����ȼ�ϵķ���
��C(s)��H2O(g) CO(g)��H2(g)����H��________kJ/mol��
CO(g)��H2(g)����H��________kJ/mol��
(2)CO
������H2O(g)��һ��������Ӧ��CO(g)��H2O(g) CO2(g)��H2(g)����H��0
CO2(g)��H2(g)����H��0
�ڼס��ҡ������������ܱ������У���ʼʱ�����±�����Ͷ�ϣ���800��ʱ�ﵽƽ��״̬��K��1.0��

�ٸ÷�Ӧ��ƽ�ⳣ������ʽΪ________��
��ƽ��ʱ����������CO��ת������________��
������CO��ת���ʣ���________�ף���________�ף�(�����������������)
�۱������У�ͨ���ı��¶ȣ�ʹCO��ƽ��ת�������ߣ����¶�________(����ߡ����͡�)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ɽ��ʡ����һ��2012������ڶ���������⻯ѧ���� ���ͣ�058
����̼ѭ�����Ѿ������˹�������ӣ��Իش��������⣺
(1)ú��������Һ���������ȼ�ϵ������ʣ�
��֪25�棬101 kPaʱ��C(s)��1/2O2(g)��CO(g)����H����126 kJ��mol��1
2H2(g)��O2(g)��2H2O(1)����H����571.6 kJ��mol��1��H2O(g)��H2O(1)����H����44 kJ��mol��1
����25�棬101 kPaʱ��C(s)��H2O(g)��CO(g)��H2(g)����H��________��
(2)��¯������CO�������Ҫ��;֮һ���������ӦΪ��
FeO(s)��CO(g)![]() Fe(s)��CO2(g)����H��0����֪��1100��ʱ���÷�Ӧ�Ļ�ѧƽ�ⳣ��K��0.263��
Fe(s)��CO2(g)����H��0����֪��1100��ʱ���÷�Ӧ�Ļ�ѧƽ�ⳣ��K��0.263��
���¶����ߣ���ѧƽ���ƶ���ﵽ�µ�ƽ�⣬��ʱƽ�ⳣ��Kֵ________(���������С�����䡱)��
��1100��ʱ��ø�¯ʱ��c(CO2)��0.025 mol��L��1��c(CO)��0.1 mol��L��1������������£��÷�Ӧ�Ƿ��ڻ�ѧƽ��״̬��________(��ǡ���)�����ж�������________��
��Ŀǰ��ҵ�Ͽ���CO2������ȼ�ϼ״����йط�ӦΪ��
CO2(g)��3H2(g)![]() CH3OH(g)��H2O(g)����H����49.0 kJ��mol��1���������Ϊ1 L���ܱ������У�����1 mol��CO2��3 mol��H2����Ӧ�����в��CO2��CH2OH(g)��Ũ����ʱ��ı仯��ͼ��ʾ��
CH3OH(g)��H2O(g)����H����49.0 kJ��mol��1���������Ϊ1 L���ܱ������У�����1 mol��CO2��3 mol��H2����Ӧ�����в��CO2��CH2OH(g)��Ũ����ʱ��ı仯��ͼ��ʾ��
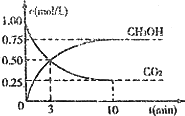
�ٴӷ�Ӧ��ʼ��ƽ�⣬������ƽ����Ӧ����v(H2)��________��
�����д�ʩ��ʹ![]() �������________(�����)��
�������________(�����)��
A�������¶�
B���ٳ���H2
C���ٳ���CO2
D����H2O(g)����ϵ�з���
E������He(g)��ʹ��ϵѹǿ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A.C8H18��l��+![]() O2(g)
O2(g)![]() 8CO2(g)+9H2O(g)����H(298 K)=-48.40 kJ��mol-1?
8CO2(g)+9H2O(g)����H(298 K)=-48.40 kJ��mol-1?
B.C8H18��l��+![]() O2(g)
O2(g)![]() 8CO2(g)+9H2O(l)����H(298 K)=-5 518 kJ��mol-1?
8CO2(g)+9H2O(l)����H(298 K)=-5 518 kJ��mol-1?
C.C8H18��l��+![]() O2(g)
O2(g)![]() 8CO2(g)+9H2O(l)����H(298 K)=+5 518 kJ��mol-1?
8CO2(g)+9H2O(l)����H(298 K)=+5 518 kJ��mol-1?
D.C8H18��l��+![]() O2(g)
O2(g)![]() 8CO2(g)+9H2O(l)����H(298 K)=-48.40 kJ��mol-1
8CO2(g)+9H2O(l)����H(298 K)=-48.40 kJ��mol-1
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com