
����Ӧ����㷺�Ľ���������±����������Լ������ĺ������ξ�Ϊ��Ҫ�����
(1)Ҫȷ������ij�Ȼ���FeClx�Ļ�ѧʽ���������ӽ����͵ζ��ķ�����ʵ���г�ȡ0.54 g��FeClx��Ʒ���ܽ���Ƚ��������ӽ���Ԥ��������ͨ�����б���OH���������ӽ�������ʹCl����OH������������������ɺ�������Һ��OH����0.40 mol��L��1������ζ��������յ�ʱ��������25.0 mL���������Ʒ���ȵ����ʵ����������FeClx��xֵ��________(�г��������)��
(2)����һ����FeCl2��FeCl3�Ļ������Ʒ�����������������n(Fe)��n(Cl)��1��2.1�������Ʒ��FeCl3�����ʵ�������Ϊ________����ʵ�����У�FeCl2�������ۺ�________��Ӧ�Ʊ���FeCl3�������ۺ�________��Ӧ�Ʊ���
(3)FeCl3������ᷴӦʱ��������ɫ���ʣ��÷�Ӧ�����ӷ���ʽΪ______��
(4)�������(K2FeO4)��һ��ǿ������������Ϊˮ��������������ز��ϡ�FeCl3��KClO��ǿ���������·�Ӧ����ȡK2FeO4���䷴Ӧ�����ӷ���ʽΪ________����MnO2��Zn������ƣ�K2FeO4��ZnҲ������ɼ��Ե�أ�K2FeO4�ڵ������Ϊ�������ϣ���缫��ӦʽΪ________���õ���ܷ�Ӧ�����ӷ���ʽΪ________��
(1)n(Cl)��0.0250 L��0.40 mol��L��1��0.010 mol,0.54 g��0.010 mol��35.5 g��mol��1��0.185 g��n(Fe)��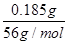 ��0.0033 mol��n(Fe)��n(Cl)��0.0033��0.010��1��3��x��3
��0.0033 mol��n(Fe)��n(Cl)��0.0033��0.010��1��3��x��3
(2)0.1��HCl��Cl2
(3)2Fe3����2I��=2Fe2����I2��(4)2Fe(OH)3��4OH����3ClO��=2FeO42-��3Cl����5H2O��
FeO42-��3e����4H2O=Fe(OH)3��5OH����
3Zn��2FeO42-��8H2O=3Zn(OH)2��2Fe(OH)3��4OH��
����


 �ǻ�С��ϰϵ�д�
�ǻ�С��ϰϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��15�֣�ʵ��̽����ѧϰ��ѧ��һ����Ҫ������ijʵ��С���ͬѧ��������װ�����һЩ���������Ʊ��Լ������������̽�����г�װ�ü���������ʡ�ԣ�����װ��E�ж����ʹ�ã���
�ɹ�ѡ���Һ���Լ�������ҩƷ��
| Һ���Լ� | ����ҩƷ |
| ϡ���ᡢϡ���ᡢϡ���ᡢNaOH��Һ��Ũ��ˮ��5%H2O2��Һ��Ũ���ᡢ����ʳ��ˮ | CaCO3��CaO��MnO2��KMnO4��CaC2�� ��ʯ�ҡ�Cu��Zn��Na2S |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������������������м�����Ҫ�����ã��������й��ա���Ч���Ͷ���ɱ�������������ǽ��չ�����ơ�
(1)Cl2��H2O2��ClO2(��ԭ����ΪCl��)��O3(1 mol O3ת��Ϊ1 mol O2��1 mol H2O)�����ʳ��������������������ʵ�����������������Ч����ߵ���________(�����)��
| A��Cl2 | B��H2O2 | C��ClO2 | D��O3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪����ǿ�������Һ���ֱ������������������еĸ�һ�֣����һ����ظ���NH4����Ba2����Na����H����SO42����NO3����OH����SO32��������������Һ�ֱ���ΪA��B��C��D����������ʵ�顣
����A��D�е���C�����г������ɣ�
��D��B��Ӧ���ɵ������ܱ�A���գ�
��A��D��Ӧ���ɵ������ܱ�B���գ�Ҳ��ʹ��ˮ��ɫ��
�Իش��������⣺
(1)D�Ļ�ѧʽ��________���ж�������___________________________________________��
(2)д�����༸�����ʵĻ�ѧʽ��A________��B________��C________��
(3)д��ʵ������йط�Ӧ�����ӷ���ʽ_______________________________��
(4)д���������ɵ���������ˮ��Ӧ�����ӷ���ʽ_______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������ͭ�Ƚ������仯�������ճ�������Ӧ�ù㷺�����������ʵ��ش����⣺
(1)�����к���һ����̼������X(Fe3C)��X�������Ŀ����и������գ������д��ԵĹ���Y����Y���ڹ����������Һ�к��еĴ�����������________��
(2)����������Ҫ�Ĺ�ҵ���ϣ��÷���м�Ʊ���������������ʾ��
�ش��������⣺
�ٲ��������õķ���������������________���������������________���ò����ľ��巽����________��
��Na2CO3��Һ���Գ����ۣ�ԭ����(�����ӷ���ʽ��ʾ)________��
����д������FeCO3���������ӷ���ʽ��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������ֿ���������A��B��C��D��E�������������������ӻ�����ͬ���ֱ�������������Na����Al3����Mg2����Ba2����Fe3��������������Cl����OH����NO3����CO32����һ�֡�
(1)ijͬѧͨ���ȽϷ�������Ϊ�������Ϳ��ж����б��е�����������________��________(�ѧʽ)��
(2)Ϊ��ȷ��X���ֽ�(1)�е��������ʼ�ΪA��B����X�����ʼ�ΪC����C��B����Һ���ʱ���������ɫ��������ɫ��ζ���壻��C��A����Һ���ʱ�����ػ�ɫ��������ó����е���ϡ������������ܽ⣬������а�ɫ���������ܽ⡣��XΪ________��
A��SO32�� B��SO4�� C��CH3COO�� D��SiO32��
(3)��CuͶ�뵽װ��D��Һ���Թ��У�Cu���ܽ⣻�ٵμ�ϡH2SO4��Cu���ܽ⣬�ܿڸ����к���ɫ������֡�������Dһ���������������е�________ (����Ӧ�����ӷ���)���йط�Ӧ�����ӷ���ʽΪ_____________________________________��
(4)���������Ѿ�ȷ�������ʣ����Լ����D��E�е������ӡ������ʵ��������衢�����ۣ�______________________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ǿ�ȸߺ��ʵ�����ŵ㣬�㷺Ӧ���ڷɻ�����ҵ�ȡ���ҵ����������������Ҫ�ɷ�Ϊ����������FeTiO3���Ʊ��ѽ�������ҵ�������£� ��֪��Ti��TiO2��ѧ�����ȶ���������ϡ���ᡢϡ����ȡ�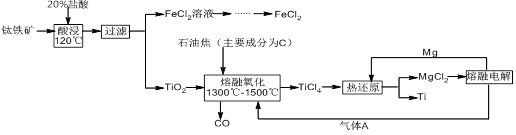
��1����д��FeTiO3��ϡ���ᷴӦ�����ӷ���ʽ�� ��
��2����д���������������Ļ�ѧ����ʽ�� ��
��3������Ȼ�þ��������ӦʽΪ�� ����ѭ�����õ�����Ϊ�� ���ѧʽ����
��4���Ȼ�ԭ�����ܷ�����ƴ���þ����ԭ���� ����ܡ����ܡ�����ԭ��Ϊ�� ��
��5���ô˷����Ʊ��õ���Ti����������MgCl2��Mg�������Լ��� ���ѧʽ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij�����Һ�п��ܺ��е��������±���ʾ:
| ���ܴ������е������� | H+��Ag+��Mg2+��Al3+��NH4+��Fe3+ |
| ���ܴ������е������� | Cl-��Br-��I-��CO32����AlO2�� |
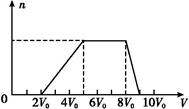
| Cl2�����(��״��) | 11.2 L | 22.4 L | 28.0 L |
| n(Cl-) | 2.5 mol | 3.5 mol | 4.0 mol |
| n(Br-) | 3.0 mol | 2.5 mol | 2.0 mol |
| n(I-) | x mol | 0 | 0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1�������������ɷ�����H1N1���С�����������Ŀǰ�����Ϲ��ϵĵ��Ĵ���Ч�����Ĺ�����������������KClO3��H2SO4��������Na2SO3��Ӧ�Ƶá���д����Ӧ�����ӷ���ʽ_____________________________________________________________________________��
��2��ij��ɫ��Һֻ��������8�������е�ij���֣�Na+��H+��Mg2+��Ag+��Cl-��OH-�� ����֪����Һ����Al2O3��Ӧ����
����֪����Һ����Al2O3��Ӧ����
�ٸ���Һ��Al2O3��Ӧ����Al3+���ɣ���ԭ��Һ��һ������_______��һ�����Ậ�д�����_______��
�ڸ���Һ��Al2O3��Ӧ����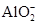 ���ɣ���ԭ��Һ��һ������_______�����ܺ��д�����_______��
���ɣ���ԭ��Һ��һ������_______�����ܺ��д�����_______��
��д������Һ��Al2O3��Ӧ����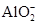 �����ӷ���ʽ____________________________��
�����ӷ���ʽ____________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com