



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

| A��a��ʱ��c(CH3COOH) >c(CH3COO��) >c(H��) >c(Na��) >c(OH��) |
| B��b��ʱ��c(Na��)��c(CH3COO��) |
| C��c��ʱ��c(H��)��c(CH3COOH)��c(OH��) |
| D��d��ʱ��c(Na��) >c(OH��) > c(CH3COO��) >c(H��) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����Ը���PbI2��AgCl��Ksp�Ĵ�С�Ƚ����ߵ��ܽ�� |
| B�������£�ͬŨ�ȵ�Na2S��NaHS��Һ��ȣ�NaHS��Һ��pH�� |
| C�������ʵ���Ũ�ȵ�CH3COONH4��Һ��NH4HSO4��Һ��ǰ�ߵ�c��NH4+���� |
| D���ö��Ե缫��ⱥ��NaCl��Һ������1.0 mol����ת�ƣ�����������1.0 mol NaOH |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
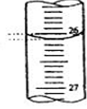
| �ζ����� | ����Һ��(mL) | �����������mL�� | |
| �ζ�ǰ����mL�� | �ζ��������mL�� | ||
| ��һ�� | 25.00 | 0.50 | 20.40 |
| �ڶ��� | 25.00 | 4.00 | 24.10 |
| ������ | 25.00 | 0.80 | 23.10 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��pH����1��HNO3��pH����13��Ba(OH)2��Һ�������Ϻ���ҺpHΪ7 |
| B��pH����3������ϡ��105������ҺpH�ӽ���7 |
| C��0.01mol/L��CH3COOH��ҺpH����2Ư���� |
D��pH����10��pH����12������NaOH��Һ�������Ϻ���Һ�е�c(H+)����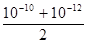 mol/L mol/L |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A��ʵ��������ͼ��ʾװ����ȡ��ϩ |
| B����ˮ�������c(H+)��10��13mol��L��1����Һ�У�Na����ClO����K����I��һ���ܴ������� |
| C����������Ҫ�ɷ��Ǹ�֬������ |
| D��ij�¶���Fe(OH)3��KSP��4��10��38������¶��£� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com