


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ����һƿŨ��Ϊ0.2mol/L��ij����Һ������Ϊ���ᡢ���ᡢ�����е�һ�֣�
����һƿŨ��Ϊ0.2mol/L��ij����Һ������Ϊ���ᡢ���ᡢ�����е�һ�֣��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��5�֣�����һƿŨ��Ϊ0.2 mol/L��ij����Һ������Ϊ���ᡢ���ᡢ�����е�һ�֡�Ϊ��ȷ��������Һ����ɽ���ʵ�飺ȡ25.00 mL0.1 mol/L������������Һ����μ��������Һ��ǡ�÷�Ӧ��ȫʱ���������Һ���Ϊ12.50 mL����ش�
��1����������� ��
��2����pH��ֽ��÷�Ӧ��������Һ�ʼ��ԣ����ݴ�����˵��������ҺΪ �������ӷ���ʽ˵����Һ�ʼ��Ե�ԭ�� ��
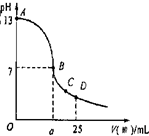
��3��ʵ���еζ���������ͼ����B�㣬a 12.5������ڡ�С�ڻ���ڣ���C�������Ũ���ɴ�С���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�걱���а�һ��ѧ�߶��ڶ�ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
��5�֣�����һƿŨ��Ϊ0.2 mol/L��ij����Һ������Ϊ���ᡢ���ᡢ�����е�һ�֡�Ϊ��ȷ��������Һ����ɽ���ʵ�飺ȡ25.00 mL0.1 mol/L������������Һ����μ��������Һ��ǡ�÷�Ӧ��ȫʱ���������Һ���Ϊ12.50 mL����ش�
��1����������� ��
��2����pH��ֽ��÷�Ӧ��������Һ�ʼ��ԣ����ݴ�����˵��������ҺΪ �������ӷ���ʽ˵����Һ�ʼ��Ե�ԭ�� ��
��3��ʵ���еζ���������ͼ����B�㣬a 12.5������ڡ�С�ڻ���ڣ���C�������Ũ���ɴ�С���� ��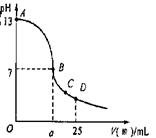
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�걱���и߶��ڶ�ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
��5�֣�����һƿŨ��Ϊ0.2 mol/L��ij����Һ������Ϊ���ᡢ���ᡢ�����е�һ�֡�Ϊ��ȷ��������Һ����ɽ���ʵ�飺ȡ25.00 mL0.1 mol/L������������Һ����μ��������Һ��ǡ�÷�Ӧ��ȫʱ���������Һ���Ϊ12.50 mL����ش�
��1����������� ��
��2����pH��ֽ��÷�Ӧ��������Һ�ʼ��ԣ����ݴ�����˵��������ҺΪ �������ӷ���ʽ˵����Һ�ʼ��Ե�ԭ�� ��
��3��ʵ���еζ���������ͼ����B�㣬a 12.5������ڡ�С�ڻ���ڣ���C�������Ũ���ɴ�С���� ��

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com