
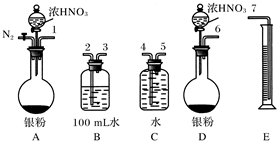
ij�С�����������ʵ�飬��ʵ��Ŀ���ǹ۲�������ȫ��ͬ�����������۵Ļ����ֱ������������NaOH��Һ��Ӧ��ɾ�̣��ⶨ�������ݶԻ����ijɷֽ��ж���������
��ش�
��1����ʯ�ҵ������� ��
��2�����в������е�˳�����ȵ������ ������ţ���
��ͬʱ��A��B�з�Һ©���Ļ������ֱ���������Լ�
�ڼ��װ�õ������� �۵�ȼ�ƾ���
��װ��ҩƷ ��Ϩ��ƾ���
��ͬʱ�ر�A��B�з�Һ©���Ļ���
��3��д��Bװ���з�����Ӧ�����ӷ���ʽ�� ��
��4��д��װ��A��B��ʵ������IJ�ͬ�� ��
��5����װ�ò������ƣ��������ӵ�װ��Ϊ ��
��6����֪��Ӧǰ����������������������Ӧ��ⶨ�����ɷֵ�������� ��
��7������Ͻ�������������������Ϊ28��27�����ַ�Ӧ��A��B����ƿ��ת�Ƶ�������֮��Ϊ ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�걱���з�̨��������ѧ��ͳһ��ϰ�����������ۣ���ѧ���� ���ͣ�ʵ����
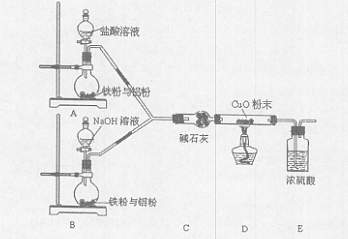
ij�С�����������ʵ�飬��ʵ��Ŀ���ǹ۲�������ȫ��ͬ�����������۵Ļ����ֱ������������NaOH��Һ��Ӧ��ɾ�̣��ⶨ�������ݶԻ����ijɷֽ��ж���������
��ش�
��1����ʯ�ҵ������� ��
��2�����в������е�˳�����ȵ������ ������ţ���
��ͬʱ��A��B�з�Һ©���Ļ������ֱ���������Լ�
�ڼ��װ�õ������� �۵�ȼ�ƾ���
��װ��ҩƷ ��Ϩ��ƾ���
��ͬʱ�ر�A��B�з�Һ©���Ļ���
��3��д��Bװ���з�����Ӧ�����ӷ���ʽ�� ��
��4��д��װ��A��B��ʵ������IJ�ͬ�� ��
��5����װ�ò������ƣ��������ӵ�װ��Ϊ ��
��6����֪��Ӧǰ����������������������Ӧ��ⶨ�����ɷֵ�������� ��
��7������Ͻ�������������������Ϊ28��27�����ַ�Ӧ��A��B����ƿ��ת�Ƶ�������֮��Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011�챱���з�̨��������ѧ��ͳһ��ϰ�����������ۣ���ѧ���� ���ͣ�ʵ����
ij�С�����������ʵ�飬��ʵ��Ŀ���ǹ۲�������ȫ��ͬ�����������۵Ļ����ֱ������������NaOH��Һ��Ӧ��ɾ�̣��ⶨ�������ݶԻ����ijɷֽ��ж���������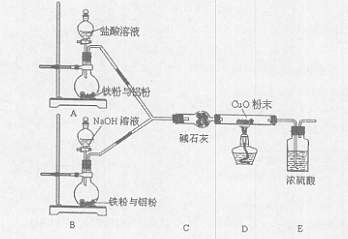
��ش�
��1����ʯ�ҵ������� ��
��2�����в������е�˳�����ȵ������ ������ţ���
��ͬʱ��A��B�з�Һ©���Ļ������ֱ���������Լ�
�ڼ��װ�õ������� �۵�ȼ�ƾ���
��װ��ҩƷ ��Ϩ��ƾ���
��ͬʱ�ر�A��B�з�Һ©���Ļ���
��3��д��Bװ���з�����Ӧ�����ӷ���ʽ�� ��
��4��д��װ��A��B��ʵ������IJ�ͬ�� ��
��5����װ�ò������ƣ��������ӵ�װ��Ϊ ��
��6����֪��Ӧǰ����������������������Ӧ��ⶨ�����ɷֵ�������� ��
��7������Ͻ�������������������Ϊ28��27�����ַ�Ӧ��A��B����ƿ��ת�Ƶ�������֮��Ϊ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com