
��1��25��ʱ��Ũ��Ϊ0.1 mol��L��1��6����Һ����HCl�� ��CH3OOH�� ��Ba(OH)2����Na 2CO3����KCl����NH4Cl��ҺpH��С�����˳��Ϊ__________________(��д���)��
��2��25��ʱ������ĵ��볣��Ka=1.7��10-5mol/L������¶���CH3COONa��ˮ��ƽ�ⳣ��Kh= mol ��L-1��������С�����һλ����
��3��25��ʱ��pH��3�Ĵ����pH��11������������Һ�������Ϻ���Һ�� ������ԡ��������ԡ����ԡ��� ����д����Һ������Ũ�ȼ��һ����ʽ�� ��
��4��25��ʱ����m mol/L�Ĵ����n mol/L������������Һ�������Ϻ���Һ��pH��7��
����Һ��c(CH3COO��) + c(CH3COOH)= ��m��n�Ĵ�С��ϵ�ǣ� �� ������������<�� ����
��5��25��ʱ����������������ʵ���Ũ�ȵĴ����백ˮ��Ϻ���Һ��pH��7 ����NH3��H2O�ĵ��볣��Ka= ��
(1)�� �� �� �� �� ��
(2)5.9��10-10
(3)���� c(Na��) + c(H��) = c(CH3COO��) + c(OH��)��
(4)m/2 mol/L ��
(5)1.7��10-5mol/L
��������
�����������1����HCl��һԪǿ�ᣬ ��CH3OOH��һԪ���ᣬ ��Ba(OH)2�Ƕ�Ԫǿ���Na 2CO3��ǿ�������Σ���KCl��ǿ��ǿ���Σ���NH4Cl��ǿ�������Ρ����ԣ�ǿ������������ǿ�������Σ����ԣ���Ĵ���ǿ�������εġ������⼸����ҺpH��С�����˳��Ϊ�� �� �� �� �� ��.
��2��KHAc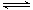 CH3COO-+H+,
CH3COO-+H+,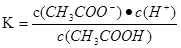 ,���¶���CH3COONa��ˮ��ƽ��ΪCH3COO-+H2O
,���¶���CH3COONa��ˮ��ƽ��ΪCH3COO-+H2O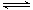 CH3COOH+OH-��
CH3COOH+OH-��
ˮ��ƽ�ⳣ��

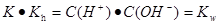
����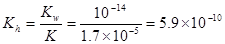 ����3��25��ʱ��pH��3�Ĵ��ᣬc(H+)=10-3mol/L,
pH��11������������Һ,c(H+)=10-11mol/L,��c(OH-)=Kw��c(H+)=10-14��10-11=10-3mol/L.������Һ�е�����Ũ����ȡ����������Ϻ���IJ����ɺ���ȫ�к͡������ڴ���Ϊ���ᡣ���д���Ϊ����Ĵ�����Ӵ��ڣ�������������H+��CH3COO-��������Һ�����ԡ�����Һ�д��ڵ���غ㡣c(Na��) + c(H��)
= c(CH3COO��) + c(OH��)����4��������ҺΪ�������ϣ�������Һ��c(CH3COO��) + c(CH3COOH)=m/2mol/L����Ϊ�������ᣬ����ǿ��������ʵ�����ϣ���ǡ������CH3COONa����Һ����CH3COO-��ˮ����Լ��ԡ�Ϊ��ʹ��Һ�����ԣ������������һЩ�����������������ˮ��ļ��ԡ�����m��n�Ĵ�С��ϵ��m��n.
��5��25��ʱ����������������ʵ���Ũ�ȵĴ����백ˮ��Ϻ���Һ��pH��7
��˵��������һˮ�ϰ���ǿ���̶���ͬ��Ҳ���ǵ���̶���ȡ����ڴ���ĵ���ƽ�ⳣ��ΪKa=1.7��10-5mol/L����NH3��H2O�ĵ��볣��Ka=1.7��10-5mol/L��
����3��25��ʱ��pH��3�Ĵ��ᣬc(H+)=10-3mol/L,
pH��11������������Һ,c(H+)=10-11mol/L,��c(OH-)=Kw��c(H+)=10-14��10-11=10-3mol/L.������Һ�е�����Ũ����ȡ����������Ϻ���IJ����ɺ���ȫ�к͡������ڴ���Ϊ���ᡣ���д���Ϊ����Ĵ�����Ӵ��ڣ�������������H+��CH3COO-��������Һ�����ԡ�����Һ�д��ڵ���غ㡣c(Na��) + c(H��)
= c(CH3COO��) + c(OH��)����4��������ҺΪ�������ϣ�������Һ��c(CH3COO��) + c(CH3COOH)=m/2mol/L����Ϊ�������ᣬ����ǿ��������ʵ�����ϣ���ǡ������CH3COONa����Һ����CH3COO-��ˮ����Լ��ԡ�Ϊ��ʹ��Һ�����ԣ������������һЩ�����������������ˮ��ļ��ԡ�����m��n�Ĵ�С��ϵ��m��n.
��5��25��ʱ����������������ʵ���Ũ�ȵĴ����백ˮ��Ϻ���Һ��pH��7
��˵��������һˮ�ϰ���ǿ���̶���ͬ��Ҳ���ǵ���̶���ȡ����ڴ���ĵ���ƽ�ⳣ��ΪKa=1.7��10-5mol/L����NH3��H2O�ĵ��볣��Ka=1.7��10-5mol/L��
���㣺��������Ũ�ȵĴ�С�Ƚϡ�����ƽ�ⳣ�����ε�ˮ��ƽ�ⳣ���Ĺ�ϵ����Һ������Ե�֪ʶ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
��֪������������ճ������м�Ϊ�������ᣬ��һ�������£�CH3COOH��Һ�д��ڵ���ƽ�⣺CH3COOH ![]() CH3COO��+H+ ����H>0
CH3COO��+H+ ����H>0
��1��25 ��ʱ��Ũ�Ⱦ�Ϊ0.1mol/L������ʹ�����Һ������˵����ȷ���� ��
������Һ��pH��ͬ
������Һ�ĵ���������ͬ
����ˮ�������c(OH��)��ͬ
 ���к͵����ʵ�����NaOH��Һ����������Һ�������ͬ
���к͵����ʵ�����NaOH��Һ����������Һ�������ͬ
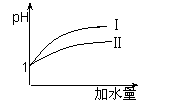
��2��25 ��ʱ����pH��Ϊ1������ʹ�����Һ�зֱ��ˮ�����ˮ
�������࣬����ҺpH�ı仯��ͼ��ʾ�����������pH�仯
�������� ��
��3��25 ��ʱ�������ΪVa mL pH=3�Ĵ�����Һ�еμ�pH=11��
NaOH��ҺVb mL����Һǡ�ó����ԣ���Va Vb���������������������
�����ʼ첿�Ź涨���۴���Ũ�Ȳ��õ���4.8g/100mL��ijͬѧ�����к͵ζ��ķ������ⶨijƷ�Ƶ�ʳ�ô��еĴ��Ậ���Ƿ��ꡣʵ����岽�����£���������ƽ��ȡһ������NaOH�����Ƴ�500mL NaOH��Һ��������֪Ũ�ȵ��������Һȷ�궨��NaOH��Һ��Ũ�ȣ�����������֪ȷŨ�ȵ�NaOH��Һ�ⶨ�����Ũ�ȡ�
��4����ֱ�������õ�NaOH��Һ�ζ���Ʒ����Ҫ�ñ������ȱ궨�ٵζ���ԭ���� ��
��5����ʵ��������£�ȷ��ȡ��ʳ�ô�20.00mL������250mL��ƿ�У��ٵμӷ�ָ̪ʾ�����ñ궨�õ�0.1000mol/L��NaOH��Һ�ζ�����ָ̪ʾ���� ɫǡ�ñ��__________ɫ�� ��Ϊ�յ㡣
�ظ��ζ���Σ������¼���£�
| �ⶨ���� | ��һ�� | �ڶ��� | ������ | ���Ĵ� |
| V��mL�� | 19.40 | 15.10 | 14.90 | 15.00 |
���ʳ�ô��д�������ʵ���Ũ�ȣ�________ mol��L��1���Ƿ�ϸ� ����ǡ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1��25��ʱ��Ũ��Ϊ0.1 mol��L��1��6����Һ����HCl�� ��CH3OOH�� ��Ba(OH)2����Na 2CO3����KCl����NH4Cl��ҺpH��С�����˳��Ϊ__________________(��д���)��
��2��25��ʱ������ĵ��볣��Ka=1.7��10-5mol/L������¶���CH3COONa��ˮ��ƽ�ⳣ��Kh= mol ��L-1��������С�����һλ����
��3��25��ʱ��pH��3�Ĵ����pH��11������������Һ�������Ϻ���Һ�� ������ԡ��������ԡ����ԡ��� ����д����Һ������Ũ�ȼ��һ����ʽ��
��
��4��25��ʱ����m mol/L�Ĵ����n mol/L������������Һ�������Ϻ���Һ��pH��7��
����Һ��c(CH3COO��) + c(CH3COOH)= ��m��n�Ĵ�С��ϵ�ǣ� �� >����������<�� ����
��5��25��ʱ����������������ʵ���Ũ�ȵĴ����백ˮ��Ϻ���Һ��pH��7 ����NH3��H2O�ĵ��볣��Ka= ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪������������ճ������м�Ϊ�������ᣬ��һ��������,CH3COOH��Һ�д��ڵ���ƽ�⣺CH3COOH ![]() CH3COO-+H+ ��H>0��
CH3COO-+H+ ��H>0��
��1��25 ��ʱ��Ũ�Ⱦ�Ϊ0.1mol/L������ʹ�����Һ������˵����ȷ���� ��
������Һ��pH��ͬ
������Һ�ĵ���������ͬ
����ˮ�������c(OH-)��ͬ
���к͵����ʵ�����NaOH��Һ����������Һ�������ͬ
��2��25 ��ʱ���� pH =5��ϡ������Һ�У�c(CH3COO-)= (�����ֱ���ʽ)��
 ��3��25 ��ʱ����pH��Ϊ1������ʹ�����Һ�зֱ��ˮ�����ˮ
��3��25 ��ʱ����pH��Ϊ1������ʹ�����Һ�зֱ��ˮ�����ˮ
�������࣬����ҺpH�ı仯��ͼ��ʾ�����������pH�仯
�������� ��
��4��25 ��ʱ�������ΪVa mLpH=3�Ĵ�����Һ�еμ�pH=11��
NaOH��ҺVb mL����Һǡ�ó����ԣ���Va���Vb��ϵ��
Va Vb�����������������������
��5��25 ��ʱ������ˮ�м���ϡ��������Һ��pH��7����ʱ[NH4��]��a mol/L��
��[Cl��]��________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com