
 Cu2+��2OH-��Ksp��c(Cu2+)��[c(OH-)]2��
Cu2+��2OH-��Ksp��c(Cu2+)��[c(OH-)]2��
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 Cu2++2OH-��Ksp=c��Cu2+��?[c��OH-��]2=2��10-20������Һ�и�����Ũ�ȷ��εij˻������ܶȻ�ʱ���������������֮�����ܽ⣮
Cu2++2OH-��Ksp=c��Cu2+��?[c��OH-��]2=2��10-20������Һ�и�����Ũ�ȷ��εij˻������ܶȻ�ʱ���������������֮�����ܽ⣮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���ܵ���� | CaCO3 | CaSO4 | MgCO3 | Mg��OH��2 |
| Ksp | 2.8��10-9 | 9.1��10-6 | 6.8��10-6 | 1.8��10-11 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ʱ�䣨s�� n��mol�� |
0 | 20 | 40 | 60 | 80 | 100 |
| n��N2O4�� | 0.40 | a | 0.20 | c | d | e |
| n��NO2�� | 0.00 | 0.24 | b | 0.52 | 0.60 | 0.60 |
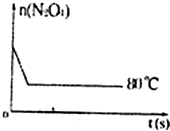


�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com