�⣺I��1��Cu�ǽ������ʣ����ܵ�������ɵ��ӣ����ڵ�KNO
3�������ƶ������ӣ��ܵ��磬�ʴ�Ϊ���٢ܣ�
��2�����ڵ�KNO
3��CuSO
4������NaOH��CaO������ȫ���룬����ǿ����ʣ��ʴ�Ϊ���ܢݢޢߣ�
��3��Һ̬SO
3��ˮ��Һ�ܵ��磬ԭ����SO
3��ˮ��Ӧ�������ᣬ�����ܵ���������ƶ����������Ӷ�ʹ��Һ���磬�����ǵ���ʣ�SO
3�Ƿǵ���ʣ��Ҵ���C
2H
5OH����ˮ��Һ�������״̬�¶����ܵ���Ļ�������ڷǵ���ʣ��ʴ�Ϊ���ڢۣ�
II����1������ɢ����ˮ��������Һʱ�����ݷ�ɢ������ֱ����С�����࣬�ѷ�ɢϵ����Ϊ����Һ��С��1nm�������壨1nm��100nm������Һ������100nm����Fe��OH��
3�����ж����ЧӦ�͵�ˮ�����ЧӦ���ʴ�Ϊ��1-100nm�����������
��2������n=

��SO
2��SO
3�����ʵ���֮��Ϊ
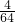
��

=1��1����ԭ�Ӻ���ԭ�Ӹ���֮��Ϊ2��5���ʴ�Ϊ��1��1��2��5��
��3��Ư�۵���ȡԭ��Ϊ2Cl
2+2Ca��OH��
2�TCa��ClO��
2+CaCl
2+2H
2O������������Һ��������Ӧ����NaClO��NaCl��H
2O����Ӧ�Ļ�ѧ����ʽΪ��Cl
2+2NaOH�TNaClO+NaCl+H
2O��
�ʴ�Ϊ��Ca��OH��
2������Ca��ClO��
2��NaClO��
������I��1�������ʵ����ԭ�������ֻҪ���������ƶ������ӻ����ɵ��Ӽ��ɣ�
��2��ǿ���������ˮ��Һ����ȫ����ĵ���ʣ�
��3���ǵ��������ˮ��Һ�������״̬�¶����ܵ���Ļ�����ö����ǰ������ǻ����
II����1���������ӵ�ֱ����С�� 1nm��100nm֮�䣬�����ЧӦ�ǽ�����������ʣ�
��2������n=

���㣻
��3��Ư�۵���ȡԭ��Ϊ2Cl
2+2Ca��OH��
2�TCa��ClO��
2+CaCl
2+2H
2O��Ư��Һ����ȡԭ��ΪCl
2+2NaOH�TNaClO+NaCl+H
2O��
���������⿼���˵������Һ�����ԭ����ʡ��ǵ���ʡ�������ʵĶ��塢�����Ļ�ѧ���ʵȣ�ע���ܵ���IJ�һ���ǵ���ʣ����������Һ������ʲ�һ���ܵ��磬������ͭ���壮ע���������ʵ�����Ħ������������֮��Ĺ�ϵ�����ʵ����ʵ�����������ԭ�ӣ������������ʵ����Ĺ�ϵ��

 ��SO2��SO3�����ʵ���֮��Ϊ
��SO2��SO3�����ʵ���֮��Ϊ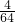 ��
�� =1��1����ԭ�Ӻ���ԭ�Ӹ���֮��Ϊ2��5���ʴ�Ϊ��1��1��2��5��
=1��1����ԭ�Ӻ���ԭ�Ӹ���֮��Ϊ2��5���ʴ�Ϊ��1��1��2��5�� ���㣻
���㣻

 ��У����ϵ�д�
��У����ϵ�д�