
����Ŀ��ij�����������Ļ�ѧʽ�ɱ�ʾΪKa[Feb(C2O4)c]��xH2O��Ϊ�ⶨ����ɣ���������ʵ�飺
����1����ȡ1.9640g������ᄃ�壬���Ƴ�250.00mL��Һ��
����2��ȡ������Һ25.00mL����ƿ�У�����1mol��L-1����5.0mL�����ȵ�70~85������0.0100mol��L-1KMnO4��Һ�ζ����յ㣬����KMnO4��Һ48.00mL��
����3����Ӧ�����Һ�м���һ����п�ۡ���������ɫǡ����ʧ�����ˣ�ϴ�ӣ������˼�ϴ��������Һ�ռ�����ƿ�У���ʱ��Һ�Գ����ԡ�
����4��������0.0100mol��L-1KMnO4��Һ�ζ�����3������Һ���յ㣬����KMnO4��Һ8.00mL��
(1)����2�У�KMnO4��C2O![]() ����ΪCO2���õζ���Ӧ�����ӷ���ʽΪ___��
����ΪCO2���õζ���Ӧ�����ӷ���ʽΪ___��
(2)����3�л�ɫ��ʧ��ԭ����___(�����ӷ���ʽ��ʾ)��
(3)�����������Һ�Ĺ����У�������ʱ��������ƿ�Ŀ̶��ߣ����������þ��������ˮ�ĺ���___(����ƫ������ƫС��������Ӱ����)��
(4)ͨ������ȷ���������������Ļ�ѧʽ___(д���������)��
���𰸡�5C2O![]() +2MnO
+2MnO![]() +16H+=10CO2��+2Mn2++8H2O 2Fe3++Zn=2Fe2++Zn2+ ƫС ���ݵζ���Ӧ5Fe2++MnO
+16H+=10CO2��+2Mn2++8H2O 2Fe3++Zn=2Fe2++Zn2+ ƫС ���ݵζ���Ӧ5Fe2++MnO![]() +8H+=5Fe3++Mn2++4H2O��250.00mL��Һ�и��������ʵ����ֱ�Ϊ��
+8H+=5Fe3++Mn2++4H2O��250.00mL��Һ�и��������ʵ����ֱ�Ϊ��
n(C2O![]() )=
)=![]() n(MnO
n(MnO![]() )��10=
)��10=![]() =0.012mol��
=0.012mol��
n(Fe3+)=n(Fe2+)=5n(MnO![]() )��10=5��0.0100mol/L��0.008L��10=0.004mol��
)��10=5��0.0100mol/L��0.008L��10=0.004mol��
���ݵ���غ��֪n(K+)=2��0.012mol-3��0.004mol=0.012mol��
���������غ�ɵ�n(H2O)=![]() =0.012mol��
=0.012mol��
n(C2O![]() )��n(Fe3+)��n(K+)��n(H2O)=0.012mol��0.004mol��0.012mol��0.012mol=3:1:3:3��
)��n(Fe3+)��n(K+)��n(H2O)=0.012mol��0.004mol��0.012mol��0.012mol=3:1:3:3��
��ѧʽΪK3[Fe(C2O4)3]��3H2O��
��������
ȡ��Ʒ���Ƴ�250.00mL��Һ��ȡ25.00mL���еζ�������Һ�ữ�����ø�����ر�Һ��C2O![]() �������������ĵĸ�����ص���ȷ����Ʒ�в����������֮�����п�۽���������ԭ���������ӣ������ø�����ر�Һ��Fe2+�������Ӷ�ȷ����Ʒ��FeԪ�ص������ٸ��ݵ���غ�ȷ�������ӵ��������������غ�ȷ��ˮ������
�������������ĵĸ�����ص���ȷ����Ʒ�в����������֮�����п�۽���������ԭ���������ӣ������ø�����ر�Һ��Fe2+�������Ӷ�ȷ����Ʒ��FeԪ�ص������ٸ��ݵ���غ�ȷ�������ӵ��������������غ�ȷ��ˮ������
(1)KMnO4��C2O![]() ����ΪCO2����������ԭ��Mn2+�����ݵ����غ�͵���غ�ɵ����ӷ���ʽΪ5C2O
����ΪCO2����������ԭ��Mn2+�����ݵ����غ�͵���غ�ɵ����ӷ���ʽΪ5C2O![]() +2MnO
+2MnO![]() +16H+=10CO2��+2Mn2++8H2O��
+16H+=10CO2��+2Mn2++8H2O��
(2)��Һ�������Fe3+���ʻ�ɫ������п�ۺ������ӷ�Ӧʹ��ɫ��ʧ�����ӷ���ʽΪ2Fe3++Zn=2Fe2++Zn2+��
(3)����ʱ��������ƿ�Ŀ̶��ᵼ�����Ƶ���ƷŨ��ƫ��ͨ���ζ���ȷ����C2O![]() ��Fe3+��K+����ƫ��ȷ��ˮ����ʱ��Ҫ����������ȥ�����������ӵ��������Իᵼ����Ʒ��ˮ�ĺ���ƫС��
��Fe3+��K+����ƫ��ȷ��ˮ����ʱ��Ҫ����������ȥ�����������ӵ��������Իᵼ����Ʒ��ˮ�ĺ���ƫС��
(4)���ݷ�Ӧ5C2O![]() +2MnO
+2MnO![]() +16H+=10CO2��+2Mn2++8H2O��֪��250.00mL��Һ��n(C2O
+16H+=10CO2��+2Mn2++8H2O��֪��250.00mL��Һ��n(C2O![]() )=
)=![]() n(MnO
n(MnO![]() )��10=
)��10=![]() =0.012mol��
=0.012mol��
MnO![]() ����Fe2+ʱ��MnO
����Fe2+ʱ��MnO![]() ת��ΪMn2+�����ϼ۽���5�ۣ�Fe2+ת��ΪFe3+�����ϼ�����1�ۣ����Զ��ߵ�ϵ����Ϊ5:1������n(Fe3+)=n(Fe2+)=5n(MnO
ת��ΪMn2+�����ϼ۽���5�ۣ�Fe2+ת��ΪFe3+�����ϼ�����1�ۣ����Զ��ߵ�ϵ����Ϊ5:1������n(Fe3+)=n(Fe2+)=5n(MnO![]() )��10=5��0.0100mol/L��0.008L��10=0.004mol��
)��10=5��0.0100mol/L��0.008L��10=0.004mol��
���ݵ���غ��֪n(K+)=2��0.012mol-3��0.004mol=0.012mol��
���������غ�ɵ�n(H2O)=![]() =0.012mol��
=0.012mol��
n(C2O![]() )��n(Fe3+)��n(K+)��n(H2O)=0.012mol��0.004mol��0.012mol��0.012mol=3:1:3:3��
)��n(Fe3+)��n(K+)��n(H2O)=0.012mol��0.004mol��0.012mol��0.012mol=3:1:3:3��
��ѧʽΪK3[Fe(C2O4)3]��3H2O��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵����ȷ����![]()
A.100mL��![]() ��HA��HB�ֱ���������п��ַ�Ӧ��HA�ų��������࣬˵��HA�����Ա�HB��
��HA��HB�ֱ���������п��ַ�Ӧ��HA�ų��������࣬˵��HA�����Ա�HB��
B.��⾫��ͭʱ���������к���Zn��Fe��Ag��Au�Ƚ���
C.��![]() ��Һ������笠���ˮ��ٽ���ˮ�ĵ��룬����ˮ�ĵ���̶�����
��Һ������笠���ˮ��ٽ���ˮ�ĵ��룬����ˮ�ĵ���̶�����
D.��һ�ܱ������з���![]() ��Ӧ������ѹǿ��ƽ��������ƶ���
��Ӧ������ѹǿ��ƽ��������ƶ���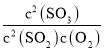 ��ֵ����
��ֵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��![]() ʱ����
ʱ����![]() ��Һ��εμӵ�
��Һ��εμӵ�![]() ��Һ�У�������Һ��
��Һ�У�������Һ��![]() ��μ�
��μ�![]() �������ϵ����ͼ��ʾ������ָ����ҺŨ�ȹ�ϵ˵����ȷ����
�������ϵ����ͼ��ʾ������ָ����ҺŨ�ȹ�ϵ˵����ȷ����
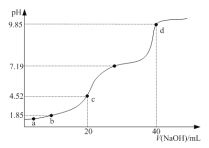
��֪��![]() ��
��![]() ʱ
ʱ![]() ��
��![]() ��
��![]() ��
��
A.![]() ��������Һ�У�
��������Һ�У�![]()
B.![]() ��������Һ�У�
��������Һ�У�![]()
C.![]() ��������Һ�У�
��������Һ�У�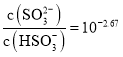
D.![]() ��������Һ�У�
��������Һ�У�![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ҹ���ѧ�Һ�°�ĸ����Ĵ����������գ������˺�°��Ƽ�ֽ�������÷��ǽ��ϳɰ�����������NH3������ƷCO2�����뱥��ʳ��ˮ��Ӧ.
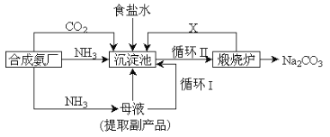
��1���÷��백��ȴ�������ԭ�������ʣ���Ҫ������_______��
��2��̼�����Ʒֽ�����Ķ�����̼��ѭ��ʹ�ã�������Ҫ���䣬�������Ҫԭ����_________����ʵ�������в��������������Ϸ�Ӧ�����������ܵ�ԭ����______��
��3������貹��Ķ�����̼һ������_________����ô�����Ƽ��貹��Ķ�����̼��������__________��
��4����°�����ȥ̼�����Ƶ�ĸҺ��ͨ����������ϸСʳ�ο�������ȴ��������Ʒ��ͨ����������____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪����A�IJ���ͨ����������һ�����ҵ�ʯ�ͻ���ˮƽ��
��![]() ��
��
����AΪ��Ҫԭ�Ϻϳɻ�����E����ϳ�·����ͼ��ʾ���ش��������⣺
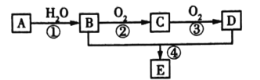
(1)д���������ʵĹ��������ƣ�B_____��C______��
(2)��Ӧ�ܵĻ�ѧ����ʽΪ_____________����Ӧ������__________��
(3)ijѧϰС�����B�Ĵ�������ʵ��װ����ͼ��ʾ���Իش��������⡣
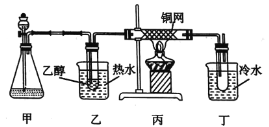
�ټ���ƿ��ʢ�ŵĹ���ҩƷ����Ϊ_______(����ĸ)��
A.![]() B.
B.![]() C.
C.![]() D.
D.![]()
��ʵ������У���װ��Ӳ�ʲ������з�����Ӧ�Ļ�ѧ����ʽΪ_____________��
������B�Ĵ����������������Ǿ�����ͬ��������Ӧ�������õ���������������������ͭ����Һ��Ϻ���ȣ�����Ϊ___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ѧѧϰС����̽����������ʣ��ü����������ʵ�飺
ʵ��1��
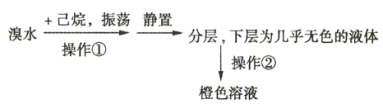
ʵ��2����������ɫ��Һװ���ܷ��Ժõ���ɫ�Լ�ƿ�С���һ��ʱ�䣬��Һ��ɫ��dz����ƿ��ƿ�ڳ��ְ�����
ʵ��3��������ͼ��ʾʵ��װ����һ�������ֽ⼺�飨���ɱ���ͱ�ϩ![]() �����ұ�ϩ�ܱ�����
�����ұ�ϩ�ܱ�����![]() ��Һ������
��Һ������
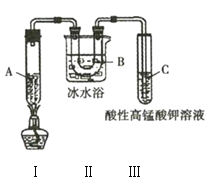
(1)ʵ��1���õ��IJ���������Ҫ��______________________________________�������ƣ���
(2)��ʵ��1��֪�����������������____________________________________��
(3)ʵ��2�еij�ɫ��Һ��dz��ԭ����__________________������ţ���
A.�������巢����ȡ����Ӧ
B.�������Ϊ��ɫ����
C.Һ������ӷ�Ũ�Ƚ���
D.������Һ�巢���˼ӳɷ�Ӧ
E.Һ���뼺��ֲ㣬�ܶȴ��Һ�����²�
(4)ʵ��3��װ�â��������______________________________________��
(5)�Թ�C�е�������______________________________________��
(6)��д��װ�â��з��������ɱ���ͱ�ϩ�Ļ�ѧ����ʽ��______________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��I.д�����з�Ӧ���Ȼ�ѧ����ʽ��
��1��CuCl(s)��O2��Ӧ����CuCl2(s)��һ�ֺ�ɫ���塣��25 ����101 kPa�£���֪�÷�Ӧÿ����1 mol CuCl(s)������44.4 kJ���÷�Ӧ���Ȼ�ѧ����ʽ��_____________________��
��2����1.01��105 Paʱ��16 g S�����������������г��ȼ�����ɶ������ų�148.5 kJ����������S����ȼ���ȵ��Ȼ�ѧ����ʽΪ________________________��
II.�о�NOx��SO2��CO�ȴ�����Ⱦ����Ĵ���������Ҫ���塣
��3��������CO��SO2�̵�����Ⱦ��һ�ַ����ǽ����ڴ���������ת��Ϊ����S���塣��֪��
��CO(g)��![]() O2(g)=CO2(g) ��H����283.0 kJ��mol��1
O2(g)=CO2(g) ��H����283.0 kJ��mol��1
��S(s)��O2(g)=SO2(g)�� ��H����296.0 kJ��mol��1
�˷�Ӧ���Ȼ�ѧ����ʽ��_____________________��
��4��������������ɹ⻯ѧ�����ͳ�������ĵ���Ҫ���塣��֪��
CO(g)��NO2(g)=NO(g)��CO2(g) ��H����a kJ��mol��1(a>0)
2CO(g)��2NO(g)=N2(g)��2CO2(g) ��H����b kJ��mol��1(b>0)
���ñ�״����3.36 L CO��ԭNO2��N2(CO��ȫ��Ӧ)������������ת�Ƶ��ӵ����ʵ���Ϊ________mol���ų�������Ϊ______________kJ(�ú���a��b�Ĵ���ʽ��ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ȿ����������̻��źŵ��ȡ���ҵ��������![]() ��
��![]() ��
��![]() ������
������![]() ���ᴿ�������¡�
���ᴿ�������¡�![]() ��֪�������ȡ����ᱵ������Ũ����
��֪�������ȡ����ᱵ������Ũ����![]()
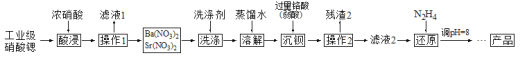
![]() Ҫ�ӿ����������������ȡ�Ĵ�ʩ��_________
Ҫ�ӿ����������������ȡ�Ĵ�ʩ��_________![]() дһ������
дһ������![]() ��
��
![]() ����1�������� _____________��ϴ�����õ�ϴ�Ӽ��� ________��
����1�������� _____________��ϴ�����õ�ϴ�Ӽ��� ________��
![]() ����Һ2���й�����
����Һ2���й�����![]() ��
��![]() ��ԭΪ
��ԭΪ![]() ��ͬʱ�ų�����Ⱦ�����壬д��������Ӧ�����ӷ���ʽ _________�����������뻹ԭ��������ʵ���֮��Ϊ _________��
��ͬʱ�ų�����Ⱦ�����壬д��������Ӧ�����ӷ���ʽ _________�����������뻹ԭ��������ʵ���֮��Ϊ _________��
![]() ��֪
��֪![]() ������ˮ����ԭ�����
������ˮ����ԭ�����![]() ��Ŀ���� ________��
��Ŀ���� ________��
![]() Ϊ�˲ⶨ������2����CrԪ�ص�������������������ʵ�顣
Ϊ�˲ⶨ������2����CrԪ�ص�������������������ʵ�顣![]() ��֪��
��֪��![]() ����
����![]()
![]() ������2����CrԪ�ص���������Ϊ _______
������2����CrԪ�ص���������Ϊ _______![]() �ô���ʽ��ʾ
�ô���ʽ��ʾ![]() ��
��
![]() �������HI��Һ����̫�࣬�ⶨ������� ________
�������HI��Һ����̫�࣬�ⶨ������� ________![]() ����ƫ��������ƫ����������Ӱ����
����ƫ��������ƫ����������Ӱ����![]() ����ԭ���� ______��
����ԭ���� ______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��![]() ��һ����Ҫ�������ܲ��ϣ���
��һ����Ҫ�������ܲ��ϣ���![]() ���ᴿ�ǹ�ҵ��������Ҫ���ڡ�ij�о���ѧϰС������˽���
���ᴿ�ǹ�ҵ��������Ҫ���ڡ�ij�о���ѧϰС������˽���![]() ���н϶��MnO��
���н϶��MnO��![]() ��Ʒת��Ϊ��
��Ʒת��Ϊ��![]() ʵ�飬���������£�
ʵ�飬���������£�
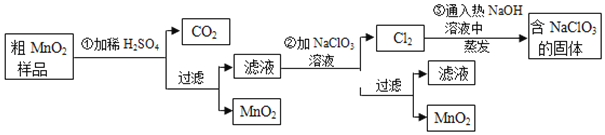
![]() ��
��![]() ����ϡ
����ϡ![]() ʱ����
ʱ����![]() ��Ʒ�е�______
��Ʒ�е�______![]() д��ѧʽ
д��ѧʽ![]() ת��Ϊ���������ʡ�
ת��Ϊ���������ʡ�
![]() ��
��![]() ����Ӧ�����ӷ���ʽ�� ______
����Ӧ�����ӷ���ʽ�� ______![]() ______
______![]() ______
______
![]() ��
��![]() �������������������������̨
�������������������������̨![]() ����Ȧ
����Ȧ![]() ��______��______��______����֪�����õ��Ĺ�������
��______��______��______����֪�����õ��Ĺ�������![]() ��NaOH����һ�����к���______
��NaOH����һ�����к���______![]() д��ѧʽ
д��ѧʽ![]() ��
��
![]() ����
����![]() ��Ʒ������Ϊ
��Ʒ������Ϊ![]() ����
����![]() ����Ӧ�����˵õ�
����Ӧ�����˵õ�![]() �����ռ���
�����ռ���![]() ��״����
��״����![]() �����ڵ�
�����ڵ�![]() ����Ӧ��������Ҫ______mol
����Ӧ��������Ҫ______mol![]() ��
��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com