
��������Դ�ı��⣬��ˮ�м����������е���ȻԪ�ء�������Դ��ѧ�����о��Ӻ�������ȡ��ѧ���ʵ�ѧ�ƣ������о��Ӻ�������ȡ����Ԫ���⣬���о��Ӻ�������ȡ��Ԫ�أ�Ũ��С��1mg/L����
��1�������к�����ߵ�±��Ԫ�������ڱ��е�λ��Ϊ ������ͬ���������ҵ���Ϊ�����Ԫ��ԭ�ӵĺ�������Ų�ʽΪ ��
��2��������Ԫ�غ���λ��ǰ�е�Ԫ���������ȡ��ơ�þ���������Ӱ뾶�Ӵ�С��˳��Ϊ �������ӷ��ű�ʾ���������γɵĻ��������ܷ���������� ���õ���ʽ��ʾ����
��3����Ԫ�����ں�ˮ����Ҫ��Be(OH)+��ʽ���ڣ�����������Ԫ�����ƣ�Ŀǰ�Ǵ��̱�ʯ����Ҫ�ɷ�Ϊ����������Be3Al2Si6O18������ȡ���������Ǻ��ա����ӡ������ȹ�ҵ���������ս�Խ������ϣ���˺�ˮ������Ϊ������Դ��ѧ�µ��о�������д����
�����������ε���������ʽ�Ļ�ѧʽ�� ��
��Be(OH)+��ǿ����Һ��Ӧ�����ӷ���ʽ�� ��
��4�����дӺ�������ȡ���ᴿ���ʵ�������ʵ�������У����������� ��ѡ���ţ���
a����ˮ���壺��ˮŨ��
 ������
������

 Һ��
Һ��
b����ˮ��þ����̲����
 ʯ����
ʯ����

 MgO
MgO þ
þ
c��������⣺��������
 ��Һ
��Һ �����л���Һ
�����л���Һ �⾧��
�⾧��
d�������ᴿ������

 ����
����

 ��Һ
��Һ
 ʳ�ξ���
ʳ�ξ���
������8�֣���1���������ڢ�A�� 1s22s22p63s23p4
��2��S2-��Cl-��O2-��Na+��Mg2+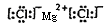
��3��3BeO��Al2O3��6SiO2 Be(OH)++3OH-��BeO22-+ 2H2O ��4��bd��2�֣�
���������������1�������к�����ߵ�±��Ԫ������Ԫ�أ���ԭ��������17�������ڱ��е�λ��Ϊ�������ڢ�A�壻����ͬ���������ҵ���Ϊ�����Ԫ����S��ԭ��������16�����ݹ���ԭ����֪Sԭ�ӵĺ�������Ų�ʽΪ1s22s22p63s23p4��
��2�����Ӳ���Խ�����Ӱ뾶Խ���ں�������Ų���ͬ�������£����Ӱ뾶��ԭ���������������С�����������ȡ��ơ�þ���������Ӱ뾶�Ӵ�С��˳��ΪS2-��Cl-��O2-��Na+��Mg2+�������γɵĻ��������ܷ�����������Ȼ�þ���������Ӽ������ӻ���������ʽ��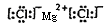 ��
��
��3����������������ʽ��ʾʱ�����ý�������������ǰ�棬Ȼ���Դ����ƣ����Ը������������εĻ�ѧʽBe3Al2Si6O18��֪������������ʽ�ɱ�ʾΪ3BeO��Al2O3��6SiO2��
��������Ԫ���Լ����������������Ԫ���Լ���������������ƣ����Ը�����������������������Һ��Ӧ�ķ���ʽ��֪��Be(OH)+��ǿ����Һ��Ӧ�����ӷ���ʽΪBe(OH)++3OH-��BeO22-+ 2H2O��
��4����������ͼ��֪��ac��ȷ����ҵ����Ȼ�þұ������þ�������ǵ������þ����Ϊ����þ���۵�̫�ߣ�b����ȷ��d���Ȼ�������������������Ba2�����ӣ����Եò����������Ȼ��ƣ�Ӧ���ȼ��Ȼ��������̼���ƣ����˺���������ữ��d����ȷ����ѡbd��
���㣺���麣ˮӦ�õ��й��жϣ�Ԫ�����ڱ��Ľṹ��Ԫ�������ɵ�Ӧ�õ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(1)���з�Ӧԭ�������Ϲ�ҵұ������ʵ���������(����)��
A��2HgO 2Hg+O2�� 2Hg+O2�� | B��Fe3O4+4CO 3Fe+4CO2 3Fe+4CO2 |
C��2MgO 2Mg+O2�� 2Mg+O2�� | D��2Ag2O 4Ag+O2�� 4Ag+O2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ʡ�Ǻ����ʡ����ˮ��һ�ַḻ����Դ����ҵ�ϴӺ�ˮ�п���ȡ���������ʣ��㷺Ӧ��������������Ƽ��ȷ��档��ͼ��ij�����Ժ�ˮ��Դ�����ۺ����õ�ʾ��ͼ��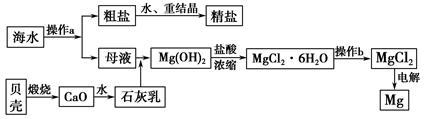
�ش��������⣺
(1)����ͼ�в���a������Ϊ_________________________________________��
(2)��ҵ�ϴӺ�ˮ����ȡ��NaCl����������ȡ������Ҫ�������£���ʳ��ˮ����ͨ������A����ͨ������B����ַ�Ӧ����˵õ�����C����ҺD��������C���ռ����Ƶô��
��������AӦ��_______________(�ѧʽ)������̷�Ӧ����ʽΪ_______________________________��
����ҺD����Ҫ����NH4Cl��NaHCO3�����ʣ���ҵ��������ҺD��ͨ��NH3��������ϸСʳ�ο�������ȴ����������NaHCO3�ĸ���ƷNH4Cl���壬��ͨ��NH3��������_______________________________________________��
(3)þ��һ����;�ܹ�Ľ������ϣ�Ŀǰ������60%��þ�Ӻ�ˮ����ȡ������b����__________�����н��У����ڿ����м��ȣ����ˮ������Mg(OH)Cl��д���йط�Ӧ�Ļ�ѧ����ʽ_________________________________________��
(4)һ�ȼÿ������234��NaCl����ЩNaCl��������������Ϊ32%���ռ���Һ__________�֣�ͬʱת��__________mol���ӡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ȫ��ˮ��ԴΣ���������أ������������Ŀ��Ͷ����ռȫ����ˮ��Լ97%�������ϣ���ӿ�ɬ�ĺ�ˮ����ȡ�������õĵ�ˮ��
(1)��ˮ���������Ѿá����δ�սʱ��һ��Ϊ�˾����;������ʵ��̴�����Ư����һ���ĵ��ϡ�Ϊ�˻�ȡ��ˮ����Ա�ǽ�����ͧ�ϵ�ͭƤ��������£��ڿ������ϼӸ�һ���Ե���ھ����γ�ˮ�۵�ͭ�ǣ�����һ���������������������ڵĺ�ˮ��ˮ�����ڸǶ�������˳�ű�Ե����ˮ�ۡ��ٵ��뵭ˮͰ�ڡ������������ֵ�������Ϊ��ͼ����������ʾ��ͼ����ͭ�ǡ�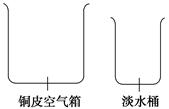
(2)��ˮ������ƺ�ˮ���Ρ��ù��չ��̰�����ˮԤ���������Ρ�����ˮ�ĺ����ȡ��������ں�ˮԤ��������________(�����)��
�ٵ�ˮ�ռ����ڳ����������ɱ��������ܳ�����ˮ�е��η֡���ˮ�ʼ�⡡���ͺ�ˮ���Ƕ�
(3)Ϊ�˼������ռ��ĵ�ˮ���Ƿ��������ӣ�ͨ�����õ��Լ���_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�Ӻ�ˮ�Ʊ�����þ��������ͼ��ʾ��
��1��Ϊ�˽�Լ�ɱ���������ú�̲��Դ���ñ��Ǿ���һϵ�з�Ӧ�����Ƶ�ʯ���飬��д���йط�Ӧ�Ļ�ѧ����ʽ��__________��__________��
��2����ʯ�����м���MgCl2��Һ����ֽ��衢���ˡ�ϴ�ӡ�д���÷�Ӧ�Ļ�ѧ����ʽ��__________��
��3�����۵���Ӳ�ȷ���������þ�Ͻ���þ��Ƚϣ����ص���__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�Ӻ�ˮ��ȡþ������������£���ش�������⡣
��1���Ӻ�ˮ����ȡþ����������ͼ��ʾ����ͼ������Ҫ�����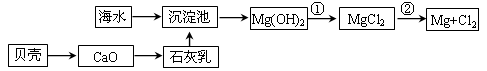
i.��ˮ���������ǰ���Ժ�ˮ���д������������ַ�����
����һ����ɹ�κ��±ˮͨ������أ�
������������������Ũ����ĺ�ˮͨ������ء�
��������________��������������_________________________________________��
ii.��Ӧ�ٵ����ӷ�����________________________________________________��
��Ӧ�ڵĻ�ѧ����ʽ��_______________________________________________��
��2���Ӻ�ˮ����ȡ�����������ͼ��ʾ����ͼ������Ҫ�����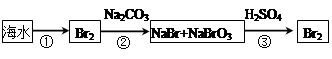

i.���̢��У�������Լ���___________��
ii.���̢��У�����Һ�д����ȿ��������崵�����ô������գ������ȿ�����Ŀ����______________________________________________________________________��
iii.���̢��з�Ӧ�Ļ�ѧ����ʽ��____________________________________________��
iv.�����յõ����嵥������Ȼ����������Cl2�����ȥ�����ʵķ�����__________________________________________________��������ӷ���ʽ�ش𣩡�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�Ͼ�Ӳ�ʺϽ��к�̼���٣�WC���������ܣ�Co�������������������õ�ⷨ�ɻ���WC��Co���������̼�ͼ���£�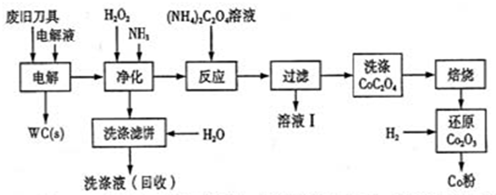
��1�����ʱ�Ͼɵ������������������������HCl��ҺΪ���Һ��������Ҫ�ĵ缫��ӦΪ ��
��2���������������˱�����Ҫ�ɷ���Fe(OH)3�����յ�ϴ��Һ����ˮ���Ƶ��Һ��Ŀ���ǻ����������е� ��
��3����ҺI����Ҫ�ɷ���NH4Cl��ϴ��CoC2O4����ֶ����ղ�Ʒ���Ȳ�������Ӱ�죬������ʱ����ɻ�����Ⱦ��ԭ���� ��
��4����Co2O3��ԭ��Co�۵Ļ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���绷�����˽���ȫ���ֹʹ��������������ˮ����������������ø�Ч����ɫ���������������ȡ�����������һ�ּ��ױ�ը��ǿ���������壬������ˮ�����ȶ����ʻ���ɫ����������ʹ��ʱ���뾡����ϡ
���������ϡ�ͣ�ͬʱ��Ҫ������ա�����ȡ�ʵ�����Ե�ⷨ�Ʊ�ClO2���������£�
��1��ClO2������ԭ��_____________����ǡ����ǡ���������8���ӽṹ����ͼ��ʾ��ⷨ�ƵõIJ�������������B��ʹʯ����Һ����ɫ����ȥ���������ѡ��_________
A������ʳ��ˮ B����ʯ�� C��Ũ���� D������ˮ
��2���ȶ��Զ���������Ϊ�ƹ�������ȶ����������Ͳ�Ʒ������˵����ȷ����( )
A���������ȿɹ㷺���ڹ�ҵ������ˮ����
B���ȶ��Զ������ȵij��ִ�������˶������ȵ�ʹ�÷�Χ
C���ڹ������ͳ�Ʒ�������ڣ�Ҫ��ͨ��װ�úͼ�⼰����װ��
��3��ŷ������Ҫ��������������Ũ�����Ʊ�����ѧ��Ӧ����ʽΪ_____________________________��ȱ����Ҫ�Dz��ʵ͡���Ʒ���Է��룬��������Ⱦ������
��4���ҹ��㷺���þ��������ϡ�͵�����������������ƣ�NaClO2����Ӧ�Ʊ�����ѧ����ʽ�� ___________________���˷����ŷ�������ŵ���____________________________��
��5����ѧ�����о�����һ���µ��Ʊ����������������ữ�IJ��ᣨH2C2O4����Һ��ԭ����
�ƣ���ѧ��Ӧ����ʽΪ______________________________________________________��
�˷���������������桢����İ�ȫ�ԣ�ԭ���� _________________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com