
�Ͼ�Ӳ�ʺϽ��к�̼���٣�WC���������ܣ�Co�������������������õ�ⷨ�ɻ���WC��Co���������̼�ͼ���£�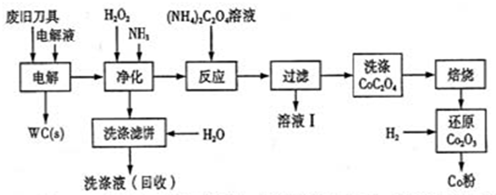
��1�����ʱ�Ͼɵ������������������������HCl��ҺΪ���Һ��������Ҫ�ĵ缫��ӦΪ ��
��2���������������˱�����Ҫ�ɷ���Fe(OH)3�����յ�ϴ��Һ����ˮ���Ƶ��Һ��Ŀ���ǻ����������е� ��
��3����ҺI����Ҫ�ɷ���NH4Cl��ϴ��CoC2O4����ֶ����ղ�Ʒ���Ȳ�������Ӱ�죬������ʱ����ɻ�����Ⱦ��ԭ���� ��
��4����Co2O3��ԭ��Co�۵Ļ�ѧ����ʽΪ ��
��1��2H++2e-=H2��
��2��Fe(OH)3 Co2+
(3) NH4C������ʱNH4Cl�ֽ�ų�NH3��HCl����
��4��3H2+Co2O3 2Co+3H2O
2Co+3H2O
������1�����ʱ�����õ����ӣ�����HCl�ǵ������Һ�����������������ӷŵ������������缫��Ӧʽ��2H++2e-=H2����
��2�����ʱ��������ʧȥ���ӣ������������ӣ�Ȼ�����˫��ˮ�����������������������ӣ��ڼ��백ˮ�����������������������������������˱�����Ҫ�ɷ���Fe(OH)3�����յ�ϴ��Һ�к���Co2+��
��3�����ڵ������Һ�����ᣬ�ڷ�Ӧ�����м����˰�ˮ�Ͳ���泥����Թ��˺���ҺI����Ҫ�ɷ���NH4Cl��ϴ��CoC2O4����֣��ḽ���Ȼ�泥����������Ȼ�立ֽ����ɰ������Ȼ��⣬�Ӷ���ɻ�����Ⱦ��
��4��������ԭCo2O3����CO�۵�ͬʱ������ˮ���ɣ���Ӧ�Ļ�ѧ����ʽ��
3H2+Co2O3 2Co+3H2O��
2Co+3H2O��


 �Ͻ�ƽСѧ��������ϵ�д�
�Ͻ�ƽСѧ��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ѧ��ѡ��2����ѧ�뼼������15�֣�
��ѧ����������Ĺؼ�����ѧΪ����������������ṩ�����ʱ�֤��
���Ļ���������Ҫ�Ļ�����Ʒ������������Ҳ���Ľ��У�������ѧ�Ҿ���Ϊ���������������ܺ������������̽����
��1��25��ʱ�ϳɰ���Ӧ�Ȼ�ѧ����ʽΪ��N2(g)+3H2(g)==2NH3(g)����H="-92.4kJ/mol" ���ڸ��¶�ʱ��ȡ1molN2��3molH2�����ܱ������У��ڴ��������½��з�Ӧ����÷�Ӧ�ų�����������С��92.4kJ����ԭ����_________________________________��
��2���������˽���ų�ֱ�Ӽ��ڵ�����������Ӧ�������ڣ��ڽϵ͵��¶Ⱥ�ѹǿ�����ºϳɰ�������˽ϺõIJ��ʡ��ӻ�ѧ��Ӧ���ʽǶȷ�������ų��Ժϳɰ���Ӧ�������� ���봫ͳ�ĺϳɰ��ķ����Ƚϣ��÷������ŵ��� ��
��(3)±ˮ���̺��ŷḻ��þ��Դ����ת����ɻ��MgCl2�ֲ�Ʒ����±ˮ����ȡþ�IJ���Ϊ��
a�������ߴ������ڵı������ճ�ʯ�ң�����ʯ���Ƴ�ʯ���飻
b����ʯ������뵽��ˮ�������о����˵õ�Mg(OH)2������
c.��Mg(OH)2�����м�������õ�MgCl2��Һ���پ������ᾧ�õ�MgCl2��6H2O��
d����MgCl2��6H2O��һ�������¼��ȵõ���ˮMgCl2��
e��������ڵ��Ȼ�þ�ɵõ�Mg��
�ٲ���d�еġ�һ��������ָ���� ��
����ͬѧ��Ϊ������b��ɼ���Mg(OH)2�õ�MgO���ٵ�����ڵ�MgO�ƽ���þ�������ɼ�ʵ�鲽�裬��ͬ���ͬѧ���뷨��?Ϊʲô?
(4) ���Ǻ˷�Ӧ����Ҫ��ȼ�ϣ��Ѿ����Ƴɹ�һ���������ӽ�����֬����ר��������ˮ�� ��U4+��������������Ԫ�ء��䷴Ӧԭ��Ϊ (��֬��HR����)���������ӽ���������ӽ���Ĥ���ᴦ�������������õ����˵���Һ���䷴Ӧԭ��Ϊ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������Դ�ı��⣬��ˮ�м����������е���ȻԪ�ء�������Դ��ѧ�����о��Ӻ�������ȡ��ѧ���ʵ�ѧ�ƣ������о��Ӻ�������ȡ����Ԫ���⣬���о��Ӻ�������ȡ��Ԫ�أ�Ũ��С��1mg/L����
��1�������к�����ߵ�±��Ԫ�������ڱ��е�λ��Ϊ ������ͬ���������ҵ���Ϊ�����Ԫ��ԭ�ӵĺ�������Ų�ʽΪ ��
��2��������Ԫ�غ���λ��ǰ�е�Ԫ���������ȡ��ơ�þ���������Ӱ뾶�Ӵ�С��˳��Ϊ �������ӷ��ű�ʾ���������γɵĻ��������ܷ���������� ���õ���ʽ��ʾ����
��3����Ԫ�����ں�ˮ����Ҫ��Be(OH)+��ʽ���ڣ�����������Ԫ�����ƣ�Ŀǰ�Ǵ��̱�ʯ����Ҫ�ɷ�Ϊ����������Be3Al2Si6O18������ȡ���������Ǻ��ա����ӡ������ȹ�ҵ���������ս�Խ������ϣ���˺�ˮ������Ϊ������Դ��ѧ�µ��о�������д����
�����������ε���������ʽ�Ļ�ѧʽ�� ��
��Be(OH)+��ǿ����Һ��Ӧ�����ӷ���ʽ�� ��
��4�����дӺ�������ȡ���ᴿ���ʵ�������ʵ�������У����������� ��ѡ���ţ���
a����ˮ���壺��ˮŨ��
 ������
������

 Һ��
Һ��
b����ˮ��þ����̲����
 ʯ����
ʯ����

 MgO
MgO þ
þ
c��������⣺��������
 ��Һ
��Һ �����л���Һ
�����л���Һ �⾧��
�⾧��
d�������ᴿ������

 ����
����

 ��Һ
��Һ
 ʳ�ξ���
ʳ�ξ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���й��ڻ�ѧ���������������ʶ�������
| A�������÷���֬�Ʒ��� |
| B��ˮ��������������ľ�ķ���� |
| C��ú��������Һ���������仯�ɱ�Ϊ���ȼ�� |
| D���������ƳɵIJ۳������ܷ�����Ũ�����Ũ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(14��)
LiPF6������ӵ���й㷺Ӧ�õĵ���ʡ�ij������LiF��PCl5Ϊԭ�ϣ����·�Ӧ�Ʊ�LiPF6�����������£�
��֪��HCl�ķе��ǣ�85.0 �棬HF�ķе���19.5 �档
��1���ڢٲ���Ӧ����ˮHF�������� �� ����Ӧ�豸�����ò������ʵ�ԭ���� (�û�ѧ����ʽ��ʾ)����ˮHF�и�ʴ�ԺͶ��ԣ�������ȫ�ֲ���ʾ�������С�Ľ�HFմ��Ƥ���ϣ���������2%�� ��Һ��ϴ��
��2��������������ˮ�����½��У��ڢ۲���Ӧ��PCl5����ˮ�⣬�����Ϊ�����ᣬд��PCl5ˮ��Ļ�ѧ����ʽ�� ��
��3���ڢܲ�������õķ����� ���ڢݲ�����β����HF��HCl���õķ����� ��
��4��LiPF6��Ʒ��ͨ����������LiF��ȡ��Ʒwg�����Li�����ʵ���Ϊnmol�������Ʒ��LiPF6�����ʵ���Ϊ mol(�ú���w��n�Ĵ���ʽ��ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ﯲ�ҵ�Ǽ��з�չDZ����ǰ�������˲�ҵ,�(Zr)Ԫ���Ǻ˷�Ӧ��ȼ�ϰ��İ�������,�������(ZrO2)�����������������մɡ��ҹ��зḻ���Ӣʯ(ZrSiO4),��Al2O3��SiO2��Fe2O3������,���������֮һ����: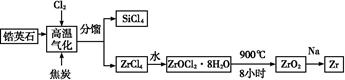
�Իش���������:
(1)д�����������и��������ķ�Ӧ����ʽ(̼ת����CO): ;
(2)д��ZrOCl2��8H2O��900 ������ZrO2�ķ�Ӧ����ʽ�� ;
(3)���ڶ�����������մɺ�ﯺϽ��˵������ȷ������������(��ѡ)��
| A��������������մ����������ǽ������� |
| B��1����=10-10�� |
| C��ﯺϽ��Ӳ�ȱȴ��Ҫ�� |
| D���ձ������˵�վ�ı�ը��������ﯺϽ��ڸ�������ˮ������Ӧ������������ը���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������ƣ�Na2FeO4�����к�ǿ�������ԣ���һ�ֱ��������õľ�ˮ����������ҵ�Ͽ���ͨ�����������������Ʊ��������ƣ������������£��ش��������⣺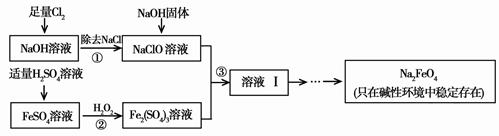
��1����������ˮ����������Ϊ������ˮ������________������ǿ�������ԣ���ɱ��������
��2������ڷ�Ӧ�����ӷ���ʽ�� ��
��3������Һ���з����Na2FeO4���и���ƷNa2SO4��NaCl��������з�Ӧ�����ӷ���ʽΪ ��
��4����һ������Na2FeO4Ͷ�뵽pH��ͬ����ˮ�У���ˮ������ɷ־���ͬ������Һ��Na2FeO4Ũ�ȱ仯��ͼ���ߢ���ʾ�����Ʋ�����II������I��Ӧ����ˮpH________����ߡ��͡�����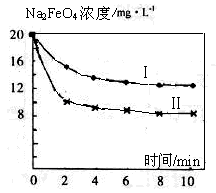
��5��ͨ�������֪Na2FeO4������Ч�ʣ��Ե�λ�����õ��ĵ�������ʾ��Լ��������_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����Ǧ�׳Ƹ��ƣ�������ˮ���㷺����Ϳ�ϡ���ī��������Ϻ��Ľ���Ʒ�ȹ�ҵ��ʵ����ģ�ҵ���ø����ࣨ����Cr2O3��Fe2O3��A12O3��SiO2�ȣ��Ʊ����ƵĹ����������£�
��1��������������Ŀ���� ������a������Ϊ ��
��2����������Ҫ�ɷ���A1(OH)3��Fe(OH)3����֪25��ʱ��A1(OH)3��Ksp=1.3��10��33������¶��·�ӦAl3++3H2O Al(OH)3+3H+��ƽ�ⳣ��Ϊ ��������λ��Ч���֣���
Al(OH)3+3H+��ƽ�ⳣ��Ϊ ��������λ��Ч���֣���
��3��д������30%H2O2�����з��������ӷ�Ӧ����ʽ�� ��
��4��ʵ����ϴ�Ӹ��Ƴ����ķ����� ��
��5��д��Ũ������A12O3��Ӧ�����ӷ���ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
ij���ϲ��������˹�̼��άΪ��ǿ�塢������Ϊ���帴�϶��ɵġ��������ֲ��Ͼ��е����ʻ���;��(����)��
�����£��ڲ����ȣ��۵��硢���ȣ��ܲ����硢�����ȣ��ݿ����ڷɻ������������ڵ����Ŀ���
| A���٢ۢݢ� | B���ڢۢݢ� |
| C���ڢۢ� | D���ۢܢ� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com