

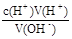 ����ʼ���ӣ��ζ��յ�ƽ�ӣ�����HCl�����������ֵ������ƫ�ߡ�������ƿ�ô���Һ��ϴ��Ȼ���ټ���10.00mL����Һ������HCl�����������ֵ������ƫ�ߡ�
����ʼ���ӣ��ζ��յ�ƽ�ӣ�����HCl�����������ֵ������ƫ�ߡ�������ƿ�ô���Һ��ϴ��Ȼ���ټ���10.00mL����Һ������HCl�����������ֵ������ƫ�ߡ�


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���٢ڢۢܢݢޢߢ� | B���٢ڢۢܢߢݢޢ� |
| C���ڢ٢ۢܢߢݢޢ� | D���ڢ٢ۢܢݢޢߢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
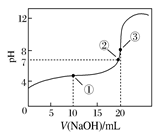
| A�������ʾ��Һ�У�c(CH3COO��)��c(OH��)��c(CH3COOH)��c(H��) |
| B�������ʾ��Һ�У�c(Na��)��c(CH3COOH)��c(CH3COO��) |
| C���ζ������п��ܳ��֣�c(CH3COOH)>c(CH3COO��)>c(H��)>c(Na��)>c(OH��) |
| D�������ʾ��Һ�У�c(Na��)>c(OH��)>c(CH3COO��)>c(H��) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����E85����������������ɵ�һ��ȼ�ϣ�������Դ |
| B���ƹ�ʹ���Ҵ�������Ϊ�˼������������ŷ� |
| C���������ġ�E85�������ͳ��ȼ�պ�ų���������� |
| D���Ҵ�������������Դ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
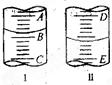
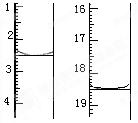
| A����amL | B����(25-a)mL |
| C��һ������amL | D��һ������(25-a)mL |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��10(a+b-12)% | B��10(a+b-14)% | C��10(12-a-b)% | D��10(14-a-b)% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��100��ʱ��pH=12��NaOH��Һ��pH=2��H2SO4ǡ���кͣ�������Һ��pH=7 |
| B��25��ʱ��0.2 mol��L Ba��OH��2��Һ��0.2 mol��L HCl�������ϣ�������Һ��pH=7 |
| C��25��ʱ��0.2 mol��L NaOH��Һ��0.2 mol��L CH3COOHǡ���кͣ�������Һ��pH=7 |
| D��25��ʱ��pH=12�İ�ˮ��pH=2��H2SO4�������ϣ�������Һ��pH>7 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com