
(14��) ��A��B��C��D��E ����Ԫ�ص�ԭ��������������B��C ����������A�������Ӻ���ԭ�ӵĵ��Ӳ�ṹ��ͬ��A��B���γ����ӻ�����B2A��C�������������ǿ�ᷴӦ��������ǿ�Ӧ��D��ԭ�ӽṹʾ��ͼΪ�� ��E�������������ǵ��Ӳ�����2�����Իش����и����⣺
��1��B��DԪ�طֱ�Ϊ �� ��
��2��DԪ��λ��Ԫ�����ڱ��е� ���ڡ��� �壻
��3��������B2A�ĵ���ʽ____________________��
��4��E������������ˮ����ķ���ʽ��________________________��
��5��D��NaOH��Һ��Ӧ�Ļ�ѧ����ʽ��_______________________________________��
��6��C����������E������������ˮ������Һ��Ӧ�����ӷ���ʽ��
_______________________________________________________________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


 ��
�� ���������ڼ��Է��ӵĽṹʽ��
���������ڼ��Է��ӵĽṹʽ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(14��)��֪A��B��C��D��E����Ԫ�����ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E��Bԭ�ӵ�p�����������γɵ��⻯��ķе���ͬ����Ԫ�ص��⻯������͵ġ�Dԭ�ӵõ�һ�����Ӻ�3p���ȫ������A+��Dԭ���γɵ�������һ�����Ӳ㡣C��A�γ�A2C�����ӻ����E��ԭ������Ϊ26��Eԭ�ӻ�������Χ�н϶���������Ŀչ��������һЩ���ӻ������γ�����
���������������ش��������⣺
��1������Ԫ��A���ε���ɫ��ӦΪ ����������ζ����Է�����ɫ��Ӧ����ԭ���� ��
��2��C���⻯����ӵļ����� ������ ���ӡ�������ԡ��Ǽ��ԡ���
��3��BD3������ԭ�ӵ��ӻ�������
��4��E��һ�ֳ��������E(CO)5�����³�Һ̬���۵�Ϊ-20.5�棬�е�Ϊ103�棬�����ڷǼ����ܼ����ݴ˿��ж�E(CO)5�ľ�������Ϊ ��
��5������E���ʵľ����ڲ�ͬ�¶��������ֶѻ���ʽ�������ֱ���ͼ��ʾ������������������������������ʵ�ʺ��е�Eԭ�Ӹ���֮��Ϊ____ ___��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010������һ�и������ڵ������¿� ���ͣ������
(14��)��֪AΪ��ɫ��Һ��B��C��I��KΪ���ʣ������Ϊ�����B��L��K ������Ϊ��ɫ��ζ���壬IΪ��ɫ�д̼�����ζ���塣GΪ��ɫ���壬F����ɫ��Ӧ����ɫ(���ܲ����۲�)����Ӧ���У�����F��P�����ʵ���֮��Ϊ1��1��������ת����ϵ����ͼ��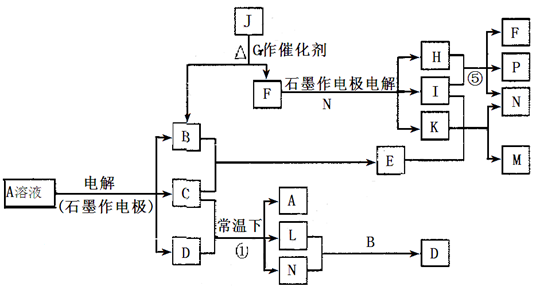
�ش��������⣺
��1��P�ĵ���ʽ��_________��I����Ԫ����Ԫ�����ڱ��е�λ����________________��
��2��д����Ӧ�ٵ����ӷ���ʽ�� ______________________________________________��
��3��M��ˮ��Һ��___________������ԡ������ԡ������ԡ����������ӷ���ʽ˵��ԭ��______________________________________________________________
��4���ö��Ե缫���400.00mL A��Һ��һ��ʱ�������ҺpH��1����ʱ��Ҫ����Һ�м���___________��������Ϊ______g������ʹ��Һ�ָ������ǰ��״̬����������Һ����仯����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��2011ѧ�꼪��ʡ����һ�и߶���ѧ�ڵ�һ���¿���ѧ�Ծ� ���ͣ������
(14��)��֪A��B��C��D��E����Ԫ�����ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E��Bԭ�ӵ�p�����������γɵ��⻯��ķе���ͬ����Ԫ�ص��⻯������͵ġ�Dԭ�ӵõ�һ�����Ӻ�3p���ȫ������A+��Dԭ���γɵ�������һ�����Ӳ㡣C��A�γ�A2C�����ӻ����E��ԭ������Ϊ26��Eԭ�ӻ�������Χ�н϶���������Ŀչ��������һЩ���ӻ������γ�����
���������������ش��������⣺
��1������Ԫ��A���ε���ɫ��ӦΪ ����������ζ����Է�����ɫ��Ӧ����ԭ���� ��
��2��C���⻯����ӵļ����� ������ ���ӡ�������ԡ��Ǽ��ԡ���
��3��BD3������ԭ�ӵ��ӻ�������
��4��E��һ�ֳ��������E(CO)5�����³�Һ̬���۵�Ϊ-20.5�棬�е�Ϊ103�棬�����ڷǼ����ܼ����ݴ˿��ж�E(CO)5�ľ�������Ϊ ��
��5������E���ʵľ����ڲ�ͬ�¶��������ֶѻ���ʽ�������ֱ���ͼ��ʾ������������������������������ʵ�ʺ��е�Eԭ�Ӹ���֮��Ϊ____ ___��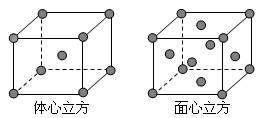
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�켪��ʡ�߶���ѧ�ڵ�һ���¿���ѧ�Ծ� ���ͣ������
(14��)��֪A��B��C��D��E����Ԫ�����ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E��Bԭ�ӵ�p�����������γɵ��⻯��ķе���ͬ����Ԫ�ص��⻯������͵ġ�Dԭ�ӵõ�һ�����Ӻ�3p���ȫ������A+��Dԭ���γɵ�������һ�����Ӳ㡣C��A�γ�A2C�����ӻ����E��ԭ������Ϊ26��Eԭ�ӻ�������Χ�н϶���������Ŀչ��������һЩ���ӻ������γ�����
���������������ش��������⣺
��1������Ԫ��A���ε���ɫ��ӦΪ ����������ζ����Է�����ɫ��Ӧ����ԭ���� ��
��2��C���⻯����ӵļ����� ������ ���ӡ�������ԡ��Ǽ��ԡ���
��3��BD3������ԭ�ӵ��ӻ�������
��4��E��һ�ֳ��������E(CO)5�����³�Һ̬���۵�Ϊ-20.5�棬�е�Ϊ103�棬�����ڷǼ����ܼ����ݴ˿��ж�E(CO)5�ľ�������Ϊ ��
��5������E���ʵľ����ڲ�ͬ�¶��������ֶѻ���ʽ�������ֱ���ͼ��ʾ������������������������������ʵ�ʺ��е�Eԭ�Ӹ���֮��Ϊ____ ___��

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com