
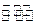 2NH3��g�� ��H=-92��44 kJ��mol���䲿�ֹ�����������ͼ��ʾ��
2NH3��g�� ��H=-92��44 kJ��mol���䲿�ֹ�����������ͼ��ʾ��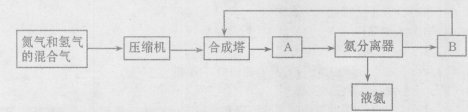
 ��������ӦΪ ��
��������ӦΪ ��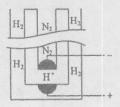
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

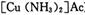 )��Һ���Ի�ô���ԭ���������У�����CO�ķ�ӦΪ��
)��Һ���Ի�ô���ԭ���������У�����CO�ķ�ӦΪ��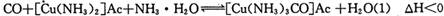
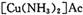 ����Ϊ
����Ϊ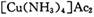 ����Ӧ�л�ԭ���������������ʵ���֮����____________��
����Ӧ�л�ԭ���������������ʵ���֮����____________��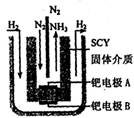
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
|
| A����ˮ | B���������� | C������ | D������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| A�������Ҵ�����(����������һ�������Ҵ�)���������ܽ��ͻ�����β�����к������ŷ� |
| B����ҵ����ʯ�����úȼ�պ��γɵ��������������������Ƶ�ʯ�� |
C��Ϊ����Ч�ķ�չ�����Դ�����õ��ˮ�ķ����� ���Ʊ�H2 ���Ʊ�H2 |
| D������ͣ������װ�������ʩ���ɽ�����β����CO��NOx��Ӧ���������� |

 ������ͼ��д����ͼ�Т٢ڵĻ�ѧʽ���� ���� ���������з����Ļ�ѧ��Ӧ����ʽΪ ��
������ͼ��д����ͼ�Т٢ڵĻ�ѧʽ���� ���� ���������з����Ļ�ѧ��Ӧ����ʽΪ ��
 4 SiHCl3��g������ƽ���H2��SiHCl3���ʵ���Ũ�ȷֱ�Ϊ0.140mol/L��0.020mol/L����H2ȫ����Դ���ȼҵ�������������Ĵ�NaCl��
4 SiHCl3��g������ƽ���H2��SiHCl3���ʵ���Ũ�ȷֱ�Ϊ0.140mol/L��0.020mol/L����H2ȫ����Դ���ȼҵ�������������Ĵ�NaCl�� ����Ϊ kg��
����Ϊ kg���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

|
 �ɡ���֪�����£�Al(OH)3��Һ��pH=3����Ksp=1��10-36��������c(Al3+)�� �� ��
�ɡ���֪�����£�Al(OH)3��Һ��pH=3����Ksp=1��10-36��������c(Al3+)�� �� �� �� ��д��ѧʽ����
�� ��д��ѧʽ���� ����ɸ��
����ɸ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
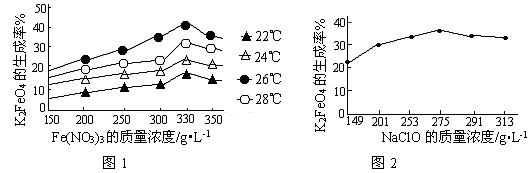
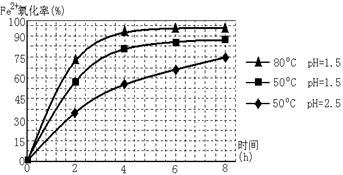
 4Fe(OH)3+8OH��+3O2�����ڡ��ᴿ��K2FeO4�в����ؽᾧ��ϴ�ӡ����º�ɵķ�������ϴ�Ӽ����ѡ��_______��Һ������ţ���
4Fe(OH)3+8OH��+3O2�����ڡ��ᴿ��K2FeO4�в����ؽᾧ��ϴ�ӡ����º�ɵķ�������ϴ�Ӽ����ѡ��_______��Һ������ţ���| A��H2O | B��CH3COONa������� | C��NH4Cl������� | D��Fe(NO3)3������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
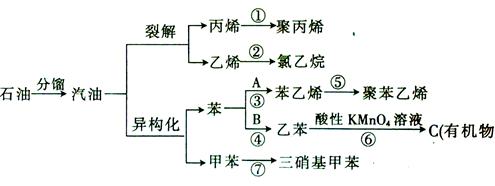
 ____________________________________________��
____________________________________________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���⣨������ | B���壨��ˮɹ�κ����Һ�� |
| C����ϩ���Ҵ��� | D��˳����ʯ���ѽ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 ��Ӧ��ƽ��ʱ������Ũ������һ���ǣ� ��
��Ӧ��ƽ��ʱ������Ũ������һ���ǣ� ��| | 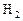 | 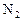 | 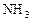 |
| A | 6 | 2 | 0 |
| B | 1 | 0 | 4 |
| C | 3.5 | 1 | 2 |
| D | 5 | 1.5 | 1 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com