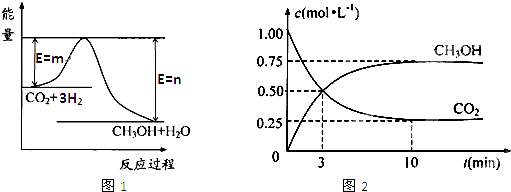
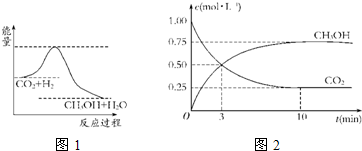
��������ЧӦ����Դ��ȱ�����⣬��ν��ʹ����е�CO
2���������Կ������ã������˸������ձ����ӣ�Ŀǰ��ҵ����һ�ַ�������CO
2����ȼ�ϼ״���һ�������·�����Ӧ��CO
2��g��+3H
2��g��?CH
3OH��g��+H
2O��g������ͼ1��ʾ�÷�Ӧ���й�������������λΪkJ?mol
-1���ı仯��
��1�����ڸ÷�Ӧ������˵���У���ȷ����
C
C
��
A����H��0����S��0�� B����H��0����S��0��
C����H��0����S��0�� D����H��0����S��0��
��2���÷�Ӧƽ�ⳣ��K�ı���ʽΪ
| c(CH3OH)��c(H2O) |
| c(CO2)��c3(H2) |
| c(CH3OH)��c(H2O) |
| c(CO2)��c3(H2) |
��
��3���¶Ƚ��ͣ�ƽ�ⳣ��K
����
����
������������䡱��С������
��4��Ϊ̽����Ӧԭ�����ֽ�������ʵ�飺�����Ϊ1L�ĺ����ܱ������У�����1molCO
2��3molH
2�����CO
2��CH
3OH��g����Ũ����ʱ�ʱ仯��ͼ2��ʾ���ӷ�Ӧ��ʼ��ƽ�⣬������Ũ�ȱ仯��ʾ��ƽ����Ӧ����v��H
2����
0.225
0.225
mol?L
-1?min
-1��
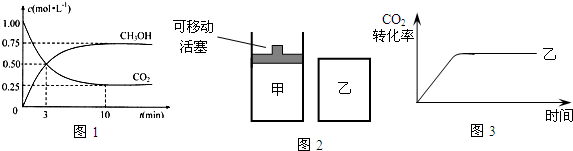
��5�����д�ʩ����ʹ��4����n��CH
3OH��/n��CO
2���������
CD
CD
��
A�������¶ȣ�
B�����������
C����H
2O��g������ϵ�з��룻
D���ٳ���1molCO
2��3molH
2��
E������He��g����ʹ��ϵ��ѹǿ����
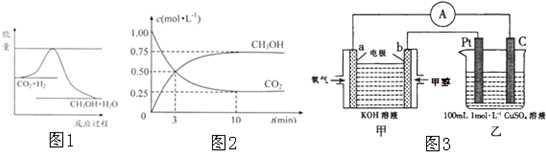
��6�����ɵļ״������Ϊȼ�ϵ�أ���ͼ3���м�װ��Ϊԭ��أ���װ��Ϊ���أ�
��b�缫�Ϸ����ĵ缫��ӦʽΪ��
CH3OH-6e-+8OH-=CO32-+6H2O
CH3OH-6e-+8OH-=CO32-+6H2O
��
����������0.1mol CH
3OH�μӷ�Ӧ������װ�������ɵ������ڱ�״���µ������Ϊ
7.84
7.84
L��





 ������CO2����ȼ�ϼ״���һ�������·�����Ӧ��CO2��g��+3H2��g��
������CO2����ȼ�ϼ״���һ�������·�����Ӧ��CO2��g��+3H2��g�� CH3OH��g��+H2O��g����������ͼ��ʾ�÷�Ӧ���й�������������λΪkJ?mol-1���ı仯��
CH3OH��g��+H2O��g����������ͼ��ʾ�÷�Ӧ���й�������������λΪkJ?mol-1���ı仯��