
����Ŀ��ijʵ��С����100mL0.50mol/LNaOH��Һ��60mL0.50mol/L��������к��ȵIJⶨ��װ����ͼ��ʾ���ش��������⣺
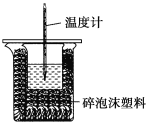
��1����ʵ�鹲��Ҫ400mLNaOH��Һ��ʵ���������Ƹ���Һʱ������Ҫ����NaOH����____g��
��2��ͼ��װ��ȱ�ٵ�������____��
��3�������Թ�����ԭ����____��
��4������д�±��е�ƽ���¶Ȳ
ʵ�� ���� | ��ʼ�¶�T1/�� | ��ֹ�¶� T2/�� | ƽ���¶Ȳ� (T2��T1)/�� | ||
HCl | NaOH | ƽ��ֵ | |||
1 | 26.2 | 26.0 | 26.1 | 30.1 | ____ |
2 | 27.0 | 27.4 | 27.2 | 33.3 | |
3 | 25.9 | 25.9 | 25.9 | 29.8 | |
4 | 26.4 | 26.2 | 26.3 | 30.4 | |
��5��������Ϊ0.50 mol/L NaOH��Һ��0.50 mol/L������Һ���ܶȶ���1 g/cm3���кͺ�������Һ�ı�����Ϊc=4.18J/(g��)������ʵ���к�����H=___��ȡС�����һλ��
��6������ʵ������57.3kJ/mol��ƫ�����ƫ���ԭ�������____
A����ȡNaOH��Һʱ���Ӷ���
B��Ϊ��ʹ��Ӧ��֣����������зִμ����
C��ʵ��װ�ñ��¸���Ч����
D����ͭ˿���沣��������
���𰸡�10.0 ���β�������� ������������Һ��ַ�Ӧ����Сʵ����� 4.0 ��53.5KJ/mol BCD
��������
����ʵ�鹲��Ҫ400 mL NaOH��Һ��ʵ���������Ƹ���Һʱ����Ҫ��500 mL����ƿ�����Ƽ�����500 mL 0.50mol/LNaOH��Һ�������ʵ���Ϊ![]() ��
��
������Ϊ![]() ���ʴ�Ϊ10.0��
���ʴ�Ϊ10.0��
��ʵ������Ҫ��װ������Ҫ���¶ȼƺͻ��β�������������ͼ��װ��ȱ�ٵ������ǻ��β�����������ʴ�Ϊ���β����������
�������Թ�����ԭ������Ҫ�ǽ�����������Һ��ַ�Ӧ����Сʵ�����ʴ�Ϊ������������Һ��ַ�Ӧ����Сʵ����
�ȸ�����ֹ�¶ȺͿ�ʼƽ���¶������һ���¶Ȳ�Ϊ4.0���ڶ����¶Ȳ�Ϊ6.1���������¶Ȳ�Ϊ3.9�����Ĵ��¶Ȳ�Ϊ4.1�������Եڶ��������Ǹ���������ݣ���ȥ��ȡ�������ε�ƽ��ֵΪ4.0���ʴ�Ϊ4.0��
�ɽ����ݴ��빫ʽ
![]()
�ʴ�Ϊ��53.5KJ/mol
��Aѡ���ȡNaOH��Һʱ���Ӷ�������ȡ������������Һ�࣬�ų��������࣬�����������ƫ��A����
Bѡ����������зִμ�������������ɢʧһ���֣��¶ȼ�������С�������������ƫС����B��ȷ��
Cѡ�ʵ��װ�ñ��¸���Ч����ų���������ɢʧһ���֣��¶ȼ�������С�������������ƫС����C��ȷ��
Dѡ���ͭ˿���沣�������裬ͭ˿�ᴫ�ȣ��ų���������ɢʧһ���֣��¶ȼ�������С�������������ƫС����D��ȷ��
������������ΪBCD��


 ���ѵ����Ԫ��ĩ���100��ϵ�д�
���ѵ����Ԫ��ĩ���100��ϵ�д� ��˼άС�ھ�100����ҵ��ϵ�д�
��˼άС�ھ�100����ҵ��ϵ�д� ��ʦָ��һ��ͨϵ�д�
��ʦָ��һ��ͨϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ͼ�б�ʾ�����й����ʣ����ɶ�����Ԫ���γɣ�֮���ת����ϵ������AΪ�����Ľ������ʣ�BΪ�ǽ������ʣ�һ���Ǻ�ɫ��ĩ����C�dz�������ɫ��ζҺ�壬D�ǵ���ɫ�Ĺ��廯���(��Ӧ����ͼ����ʡ��)��
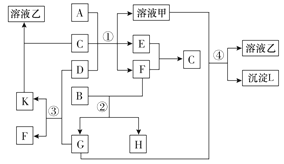
(1)A��C���������ʷֱ�Ϊ______��______(�ѧʽ)��
(2)��Ӧ���е�C��D���������÷�Ӧ�Ļ�ѧ����ʽ�� _____________��
(3)��Ӧ���У���B��F���ʵ���֮��Ϊ4��3��G��H���ʵ���֮��Ϊ______��
(4)��Ӧ�ܵ����ӷ���ʽΪ______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������Ȼ���е������٣���;�dz��㷺��ͨ���Ի����Ϊԭ����ȡ�����飬����������ͼ��ʾ��
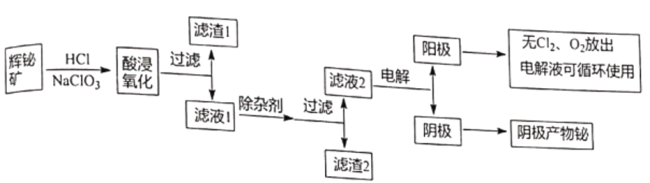
��֪���ٻ������Ҫ�ɷ���Bi2S3����������Bi2O3��SiO2�����������������ȡ�
��Bi2O3�������ᣬNaBiO3������ˮ��
�۳����£�Ksp[Fe(OH)3]=4��10-38,Ksp[Bi (OH)3]=4��10-30��Ksp[Fe(OH)2]=8.0��10-16��
�ش��������⣺
��1��д���������ʱBi2S3�����������ʵĻ�ѧ����ʽ_______________��
��2������1�ijɷ�Ϊ_______________��
��3�����Ӽ�������a.������ҺpH,b_______________��д��һ������߲�������ij��Ӽ�_______________��
��4����Һ2���������Ʊ�NaBiO3,������Һ2�м���NaOH��NaClO��Һ��ȡNaBiO3,д���÷�Ӧ�����ӷ���ʽ_______________��
��5����Һ2���õ�ⷨ��ȡ�����鵥�ʣ��������ﴦ����ɼ���ѭ��ʹ�ã����װ����ͼ��ʾ��
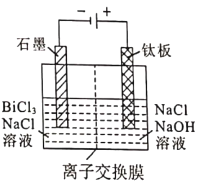
�ٽ���Ĥ����Ϊ_______________���Cl-����OH-��)����Ĥ��
�������缫��ӦʽΪ_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£���50mL����0.1molCl2����ˮ�еμ�2mol��L-1��NaOH��Һ���õ���ҺpH������NaOH��Һ����ı仯ͼ����ͼ��ʾ������˵����ȷ���ǣ� ��
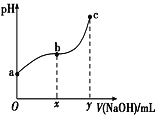
A.a����Һ�д��ڣ�N(HClO)+N(Cl-)+N(ClO-)=0.2NA(N��ʾ������������������a����Һ��Ư������������Һ�м���̼��ƹ���
B.��a��pH=4����c(Cl��)=m��c(ClO-)����Ka(HClO)=![]()
C.b��c�Σ���NaOH��Һ�ĵ��룬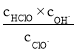 ������
������
D.��y=200����c���Ӧ��Һ�У�c(HClO)+c(H+)=c(OH-)��2c(Cl-)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijͬѧ������ͼװ�ý���ʵ�飬֤����ͭ��ϡ���ᷴӦ������NO��ʵ��ʱ������ע�����ڼ���һ������ϡ���ᣬ�ž�ע�����ڵĿ�����Ѹ�ٽ�����ͭ˿����Ƥñ���ϣ�һ��ʱ���ע����������ɫ���������
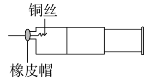
��1��ͭ��ϡ���ᷴӦ�Ļ�ѧ����ʽΪ__��
��2��NO�Ǵ�����Ⱦ��֮һ��Ŀǰ��һ��������������400�����ҡ��д������ڵ�����£��ð�����NO��ԭΪ������ˮ���÷�Ӧ�Ļ�ѧ����ʽΪ4NH3+6NO![]() 5N2+6H2O��ÿ��10molN2���ɣ����������뻹ԭ��������ʵ���֮��Ϊ__��
5N2+6H2O��ÿ��10molN2���ɣ����������뻹ԭ��������ʵ���֮��Ϊ__��
��3��N2O4Ϊ����������������ȼ��ƫ�����£�C2H8N2����Ӧ���ų����������ѻ������̫�գ�ͬʱ�������������壬��Ӧ�Ļ�ѧ����ʽΪ__��
��4����д��ʵ������NH4Cl��Ca(OH)2������ȡ�����Ļ�ѧ����ʽ__��
�ڽ�����Ȫʵ�飬����ˮ�м����̪���õ���ɫ��Ȫ����ʵ�������˰�����������Ҫ���ʷֱ���__��__��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ҵ�Ϻϳɰ���Ӧ�������仯��ͼ��ʾ���÷�Ӧ���Ȼ�ѧ����ʽ��
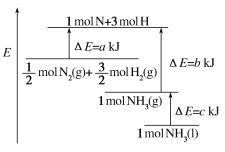
A. N2(g)��3H2(g)=2NH3(l) ��H��2(a��b��c)kJ��mol��1
B. N2(g)��3H2(g)=2NH3(g) ��H��2(b��a)kJ��mol��1
C. ![]() N2(g)��
N2(g)��![]() H2(g)=NH3(l) ��H��(b��c��a)kJ��mol��1
H2(g)=NH3(l) ��H��(b��c��a)kJ��mol��1
D. ![]() N2(g)��
N2(g)��![]() H2(g)=NH3(g) ��H��(a��b)kJ��mol��1
H2(g)=NH3(g) ��H��(a��b)kJ��mol��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʵ��װ�ô�����ǣ� ��
A.��Ȳ����ȡ���ռ� B.����������Ӧ
B.����������Ӧ
C.������������ȡ D.��ϩ����ȡ
D.��ϩ����ȡ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijѧ����NaOH����Һ�ζ�δ֪Ũ�ȵ����ᣬ����������ʵ�飺
��ʵ�鲽�裺������գ�
��1���ζ�����ʹ��ǰӦ�ȼ����Ƿ�©Һ��Ȼ��������ˮϴ�ӣ������____��ϴ��
��2��������������Һװ��ζ����ų����ݲ�����Һ�档���Һ���ʼλ����ͼ��ʾ�����ʱ�Ķ���Ϊ____mL��
![]()
��3��ȡ15.00mL��������װ����ƿ�У��μ�2�η�̪��ָʾ�����ζ��DZߵα�ҡ����ƿ���۾�Ӧ�۲�____��ѡ���ţ���
a���ζ�����Һ��ı仯 b����ƿ����Һ��ɫ�ı仯
��ʵ���¼��
ʵ����� | �������������mL�� | ������������Һ�����mL�� | ||
������ | ĩ���� | ������� | ||
1 | 15.00 | 0.50 | 17.75 | ____ |
2 | 15.00 | 0.05 | 16.10 | 16.05 |
3 | 15.00 | 0.00 | 15.95 | 15.95 |
��4������д1�鷴Ӧ���ĵ�����������Һ�����
�����ݴ��������ۣ�
��5����������ʱӦ��ȥ������Թ�����쳣���ݣ����µ�������NaOH��Һ��ƽ������ֵ��___mL����NaOH����Һ��Ũ��Ϊ0.1020mol/L���������Ũ��Ϊ___mol/L��
��6���ڱ�ʵ������У����в����������ʵ��������___��ѡ���ţ���
a����ƿ�м��������Һ���ټ���������ˮ
b����ƿ�ڵζ�ʱ����ҡ����������Һ�彦��
c������ָʾ������ɫ�б仯��ֹͣ�ζ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ݻ�һ�����ܱ������У����淴ӦA(g)+B(g)![]() xC(g)������ͼ��ʾ�Ĺ�ϵ���ߣ�������ͼ�����ж���ȷ���ǣ� ��
xC(g)������ͼ��ʾ�Ĺ�ϵ���ߣ�������ͼ�����ж���ȷ���ǣ� ��
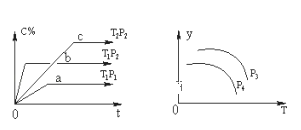
A.p3��p4��y���ʾA�����ʵ�������
B.������A��Ũ�ȣ�ƽ����ϵ��ɫ���Cһ��������ɫ������
C.p3��p4��y���ʾƽ�ⳣ��K
D.p3>p4��y���ʾ��������ƽ��Ħ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com