��ͼ1��Ԫ�����ڱ���һ���֣�
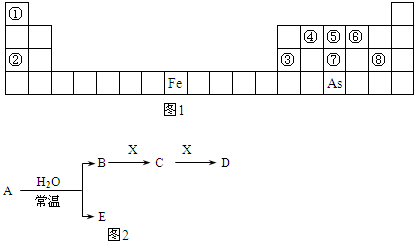
���û�ѧ����ش��������⣺
��1���ڡ��ޡ�������Ӱ뾶�ɴ�С��˳��Ϊ
Cl����O2����Na+
Cl����O2����Na+
��
��2���ܡ��ߡ������ۺ������������ǿ������˳����
HClO4��H3PO4��H2CO3
HClO4��H3PO4��H2CO3
��
��3��As��ԭ�ӽṹʾ��ͼΪ
�����⻯��Ļ�ѧʽΪ
AsH3
AsH3
��
��4��Y�ɢڢޢ�����Ԫ����ɣ�����ˮ��Һ�������г�������������As����Y��ˮ��Һ��Ӧ��������As����ۺ����ᣬ�÷�Ӧ�Ļ�ѧ����ʽΪ
5NaClO+2As+3H2O�T2H3AsO4+5NaCl
5NaClO+2As+3H2O�T2H3AsO4+5NaCl
��������1mol��ԭ��ʱ������ת����
5
5
mol��
��A��B��C��D��E��X���������ڱ�����Ԫ����ɵij������ʻ����֪A��B��C��D��E��X������ͼ2��ʾת����ϵ������������ͷ�Ӧ������ȥ����
��������AΪ����ɫ���壬BΪǿ�ᣬXΪ������������
��5��A��ˮ��Ӧ�Ļ�ѧ����ʽΪ
3NO2+H2O=2HNO3+NO
3NO2+H2O=2HNO3+NO
��
��6����ҵ�ϳ����Ȼ�ԭ��ұ��X��д���仯ѧ����ʽ
��
��7��ij�¶��£���100�棩��m��X��H
2O��Ӧ�ų�QKJ ��Q��O����������д���÷�Ӧ���Ȼ�ѧ����ʽ
3Fe��s��+4H
2O��g���TFe
3O
4��s��+4H
2��g����H=-
kJ/mol
3Fe��s��+4H
2O��g���TFe
3O
4��s��+4H
2��g����H=-
kJ/mol
��
��8������X��B��ϡ��Һ��Ӧ����C�����ӷ�Ӧ����ʽΪ
Fe+4H++NO3��=Fe3++NO��+2H2O
Fe+4H++NO3��=Fe3++NO��+2H2O
��


 ˮ�ĵ���ƽ��������ͼ��ʾ��
ˮ�ĵ���ƽ��������ͼ��ʾ�� ˮ�ĵ���ƽ��������ͼ��ʾ��?
ˮ�ĵ���ƽ��������ͼ��ʾ��?

