
 �����谷���ṹ��ʽ���ң����ں������߶�����������������ʳƷ���Ӽ�����������谷Ҳ���˳�Ϊ���������� �����谷��һ�ִ���ɫ���壬��ζ����ѹ�۵�354�棨�ֽ⣩�����ټ��������������¶�300�森�����谷���ںϳ�ʹ��˫�谷�����ɵ�ʯ��CaC2����N2��Χ�м��ȿ��Ʊ��谷���ƣ�CaCN2�����谷����ˮ����������˫�谷��C2H4N4�����ټ��ȼ�ת��Ϊ�����谷����÷���ȣ����ط��ɱ��ͣ�Ŀǰ�϶���ã���������Ϊ���壬�轺Ϊ��������380-400���¶��·��ڷ�Ӧ���������谷��6CO��NH2��2��C3N6H6+6NH3+3CO2��
�����谷���ṹ��ʽ���ң����ں������߶�����������������ʳƷ���Ӽ�����������谷Ҳ���˳�Ϊ���������� �����谷��һ�ִ���ɫ���壬��ζ����ѹ�۵�354�棨�ֽ⣩�����ټ��������������¶�300�森�����谷���ںϳ�ʹ��˫�谷�����ɵ�ʯ��CaC2����N2��Χ�м��ȿ��Ʊ��谷���ƣ�CaCN2�����谷����ˮ����������˫�谷��C2H4N4�����ټ��ȼ�ת��Ϊ�����谷����÷���ȣ����ط��ɱ��ͣ�Ŀǰ�϶���ã���������Ϊ���壬�轺Ϊ��������380-400���¶��·��ڷ�Ӧ���������谷��6CO��NH2��2��C3N6H6+6NH3+3CO2��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
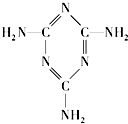 2008��9�·ݣ������ʼ��ֲܾ�����֪��Ʒ���̷��к��������谷������������������ʯ����֪�����谷�Ľṹ��ʽ��ͼ��ʾ���������������˵����ȷ���ǣ�������
2008��9�·ݣ������ʼ��ֲܾ�����֪��Ʒ���̷��к��������谷������������������ʯ����֪�����谷�Ľṹ��ʽ��ͼ��ʾ���������������˵����ȷ���ǣ��������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����谷�Ľṹ��ʽ���ң�
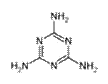 �������谷�ķ���ʽΪ____________��������̼ԭ�ӵ��ӻ���ʽ��______�������д���ͬһƽ��ĵ�ԭ����______����
�������谷�ķ���ʽΪ____________��������̼ԭ�ӵ��ӻ���ʽ��______�������д���ͬһƽ��ĵ�ԭ����______����
�������谷��ˮ��Һ�������ԣ���ԭ����_______________________________________��
�������谷�ڸ����¿��ͷ��軯��������������[Fe(CN)64-]��Ҳ����CN-��д��һ����CN-��Ϊ�ȵ�����ĵ��ʷ��ӵ�·��˹�ṹʽ________________��
�������谷��״Ϊ����ɫ��б�⾧�壬��ζ���ܶ�1.573 g��cm3 (16 ��)����ѹ�۵�354 �棨�ֽ⣩�����ټ��������������¶�300 �档�ݴ˿��ж������谷�ľ�������ӦΪ______________���塣
�ɵ����ʵĻ����ṹ��ԪΪ�����ᣬ�京����һ�㲻����30%������ʳƷ�����Ϲ�ҵ�����ʺ������Է�����ȱ��(�Բⶨ��������ȷ�������ʵĺ���)���������谷��������������ʳƷ���Ӽ��������ʳƷ����еĵ����ʺ���ָ�꣬�������谷���˳�Ϊ����������ͨ������˵�������谷����Ϊ���������ɡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���Ĵ�ʡ�����и߶���ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ������
��9�֣�ʳƷ��ȫ��ϵ����������Ӱ��ʳƷ��ȫ�����غܶࡣ
��1����ƫ������ϩ�Ľṹ��ʽΪ �����г�ǿ��������ܣ�����Ϊ����ʳƷ�İ�װ���ϣ������� ��д�ṹ��ʽ�������Ӿ۷�Ӧ�Ƶõġ�
�����г�ǿ��������ܣ�����Ϊ����ʳƷ�İ�װ���ϣ������� ��д�ṹ��ʽ�������Ӿ۷�Ӧ�Ƶõġ�
��2���پ��������״��������꣬�����Ҵ��ͼ״��Ļ��Һ�з�����״������з�����ȷ���ǣ�����ţ� ��
�ٹ��� �ڷ�Һ ������ �ܽᾧ
��3�����̷��г����к����������谷���ṹ��ʽ��ͼ��ʾ������֪1 g�����谷������ȫȼ������N2��CO2��Һ̬ˮʱ���ų�a kJ������1 mol H2O(g)ת��Ϊ1 mol H2O(l)ʱ�ų�44 kJ����������101 kPa 120�������谷������ȫȼ��ʱ����N2��CO2��ˮ�������Ȼ�ѧ����ʽΪ��

��4������ֲ�����е������ᣨC18H32O2�������ܵͣ������й��������������ȷ���ǣ�����ţ� ��
������������еĺ���������Ϊ�Ȼ�
���������������ȫӲ��ʱ������H2�����ʵ���֮��Ϊ1��2
��������ɷ���������Ӧ���ӳɷ�Ӧ��ȡ����Ӧ
�ܿ�������KMnO4��Һ�����������������������Ӧ�Ƿ���ȫ
��5���ڵ����м�������Ƶ÷�˿�ж��������ֳƵ�ۣ���ѧ����Ϊ��ˮ�ϴ��������Ƽ�ȩ����ѧʽΪ��NaHSO2��CH2O��2H2O����Է�������Ϊ��154������Ư�����á���֪ij�ֺ�����ķ�˿�������ǵ������ʧ��1 g�к���0.32 %����ÿǧ�����ַ�˿�к����������Ϊ g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013���Ĵ�ʡ�����и߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ������
��9�֣�ʳƷ��ȫ��ϵ����������Ӱ��ʳƷ��ȫ�����غܶࡣ
��1����ƫ������ϩ�Ľṹ��ʽΪ
 �����г�ǿ��������ܣ�����Ϊ����ʳƷ�İ�װ���ϣ�������
��д�ṹ��ʽ�������Ӿ۷�Ӧ�Ƶõġ�
�����г�ǿ��������ܣ�����Ϊ����ʳƷ�İ�װ���ϣ�������
��д�ṹ��ʽ�������Ӿ۷�Ӧ�Ƶõġ�
��2���پ��������״��������꣬�����Ҵ��ͼ״��Ļ��Һ�з�����״������з�����ȷ���ǣ�����ţ� ��
�ٹ��� �ڷ�Һ ������ �ܽᾧ
��3�����̷��г����к����������谷���ṹ��ʽ��ͼ��ʾ������֪1 g�����谷������ȫȼ������N2��CO2��Һ̬ˮʱ���ų�a kJ������1 mol H2O(g)ת��Ϊ1 mol H2O(l)ʱ�ų�44 kJ����������101 kPa 120�������谷������ȫȼ��ʱ����N2��CO2��ˮ�������Ȼ�ѧ����ʽΪ��

��4������ֲ�����е������ᣨC18H32O2�������ܵͣ������й��������������ȷ���ǣ�����ţ� ��
�� ����������еĺ���������Ϊ�Ȼ�
�� �������������ȫӲ��ʱ������H2�����ʵ���֮��Ϊ1��2
�� ������ɷ���������Ӧ���ӳɷ�Ӧ��ȡ����Ӧ
�� ��������KMnO4��Һ�����������������������Ӧ�Ƿ���ȫ
��5���ڵ����м�������Ƶ÷�˿�ж��������ֳƵ�ۣ���ѧ����Ϊ��ˮ�ϴ��������Ƽ�ȩ����ѧʽΪ��NaHSO2��CH2O��2H2O����Է�������Ϊ��154������Ư�����á���֪ij�ֺ�����ķ�˿�������ǵ������ʧ��1 g�к���0.32 %����ÿǧ�����ַ�˿�к����������Ϊ g��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com