
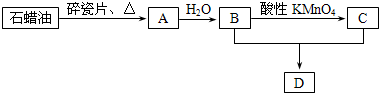
(17��)��A��B��C��D��E 5��Ԫ�أ����ǵĺ˵�������������Ҷ�С��20������C��E�ǽ���Ԫ�أ�A��E��ͬһ�壬����ԭ�ӵ����������Ų�Ϊns1��B��DҲ��ͬһ�壬����ԭ��������p�ܼ���������s�ܼ���������������Cԭ��������ϵ���������Dԭ��������ϵ�������һ�롣��ش��������⣺
��1��A��________��B��________��E��_________��
��2��д��CԪ�ػ�̬ԭ�ӵĵ����Ų�ʽ_________________________��
��3��B ��D����Ԫ�ص��⻯���У��е����� ��д��ѧʽ�ţ�����ԭ����
��4��Ԫ��B��D�ĵ縺�ԵĴ�С��ϵ��___________��C��E�ĵ�һ�����ܵĴ�С��ϵ��___________�����>������<����=������Ԫ�ط��ű�ʾ��
��5��Eλ�����ڱ��ĵ� ���ڣ��� �壻��ԭ�ӽṹʾ��ͼΪ ��
��6��A��B�γɵ�A2B2�����ﺬ ��������ԡ��Ǽ��ԡ������� ���ӡ�������ԡ��Ǽ��ԡ��������ʽΪ:______________________________��
 �п������п��Ծ����ϵ�д�
�п������п��Ծ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| Cu |
| �� |
| Cu |
| �� |
| Ũ���� |
| �� |
| Ũ���� |
| �� |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(17��)��A��B��C��D��E���ֶ�����Ԫ�أ���֪���ڵ�A��B��C��D����Ԫ��ԭ�Ӻ����56�����ӣ������ڱ��е�λ����ͼ��ʾ��E�ĵ��ʿ����ᷴӦ��1molE���������������ã��ڱ�״�����ܲ���33.6LH2��E����������A�������Ӻ�����Ӳ�ṹ��ȫ��ͬ���ش��������⣺
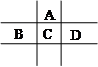
������Ԫ�ص����ƣ�A__________��B______________��C____________��D_____________��E______________��
(2)E������������ˮ�����NaOH��Ӧ�����ӷ���ʽ��__________________________________________________
(3) ��C�IJ��ȶ��������D�ĵ��ʵ����ʵ���ͬʱͨ�뵽Ʒ����Һ�У��۲쵽��������___________________________���йط�Ӧ�����ӷ���ʽΪ________________��
��4�������һ����ʵ��Ƚ�A��C�ķǽ����Ե�ǿ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013���Ĵ�ʡ�ɶ��и߶�10�¼�⻯ѧ�Ծ� ���ͣ������
(17��)��A��B��C��D��E 5��Ԫ�أ����ǵĺ˵�������������Ҷ�С��20������C��E�ǽ���Ԫ�أ�A��E��ͬһ�壬����ԭ�ӵ����������Ų�Ϊns1��B��DҲ��ͬһ�壬����ԭ��������p�ܼ���������s�ܼ���������������Cԭ��������ϵ���������Dԭ��������ϵ�������һ�롣��ش��������⣺
��1��A��________��B��________��E��_________��
��2��д��CԪ�ػ�̬ԭ�ӵĵ����Ų�ʽ_________________________��
��3��B ��D����Ԫ�ص��⻯���У��е����� ��д��ѧʽ�ţ�����ԭ����
��4��Ԫ��B��D�ĵ縺�ԵĴ�С��ϵ��___________��C��E�ĵ�һ�����ܵĴ�С��ϵ��___________�����>������<����=������Ԫ�ط��ű�ʾ��
��5��Eλ�����ڱ��ĵ� ���ڣ��� �壻��ԭ�ӽṹʾ��ͼΪ ��
��6��A��B�γɵ�A2B2�����ﺬ ��������ԡ��Ǽ��ԡ������� ���ӡ�������ԡ��Ǽ��ԡ��������ʽΪ:______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�����ʡ֣���и�һ��ѧ�ڵ�һ���¿���ѧ�Ծ� ���ͣ������
(17��)��A��B��C��D��E���ֶ�����Ԫ�أ���֪���ڵ�A��B��C��D����Ԫ��ԭ�Ӻ����56�����ӣ������ڱ��е�λ����ͼ��ʾ��E�ĵ��ʿ����ᷴӦ��1molE���������������ã��ڱ�״�����ܲ���33.6LH2��E����������A�������Ӻ�����Ӳ�ṹ��ȫ��ͬ���ش��������⣺
������Ԫ�ص����ƣ�A__________��B______________��C____________��D_____________��E______________��
(2)E������������ˮ�����NaOH��Ӧ�����ӷ���ʽ�� __________________________________________________
(3) ��C�IJ��ȶ��������D�ĵ��ʵ����ʵ���ͬʱͨ�뵽Ʒ����Һ�У��۲쵽��������___________________________���йط�Ӧ�����ӷ���ʽΪ________________��
��4�������һ����ʵ��Ƚ�A��C�ķǽ����Ե�ǿ����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com