
����Ŀ�����������ڹ�ҵ�����Ѓӹ㷺��Ӧ�ã�ijͬѧ��ʵ���ж��������ε��Ʊ������ʽ���̽����
(1)Cu2SO3��CuSO32H2O��һ�����ɫ���壬������ˮ���Ҵ���100��ʱ�����ֽ⣬���Ʊ�ʵ��װ����ͼ��ʾ��

������X��������________����������װ��A��ȡSO2ʱ���ý�Ũ�����������ϡ���ᣬ��ԭ����____________________��
��װ��C��������________________________��
��װ��B�з�����Ӧ�����ӷ���ʽΪ_____________________��
����װ��B�л�õĹ�������������ˮ���ϴ�ӣ�����ո������ֱ���ú�ɵķ�ʽ�õ���Ʒ����ԭ����_________________________��
(2)��NaHSO3��Һ�м���NaClO��Һʱ����Ӧ�����ֿ��ܵ������
I.NaHSO3��NaClOǡ�÷�Ӧ��II.NaHSO3������III.NaClO��������ͬѧ��ͨ������ʵ��ȷ���÷�Ӧ������һ�������������±���
ʵ����� | Ԥ�������� |
ȡ������Ӧ��Ļ����Һ���Թ� A�У��μ�ϡ���� | �������ݲ�������_��__(�I����II����III������ͬ)��������û�����ݲ�������_��___���� |
��ȡ������Ӧ��Ļ����Һ���Թ�B�У��μӼ��ε���KI��Һ�� ����� | ��___����III���� |
(3)����Ƽ�ʵ�鷽���Ƚ�������NaHSO3ŨҺ��HSO3-�ĵ���ƽ�ⳣ��Ka��ˮ��ƽ�ⳣ��Kb����Դ�С��________________________��
���𰸡� ��Һ©�� SO2������ˮ���ý�Ũ������������SO2���ݳ� ��ֹ����(������ȫƿ) 3Cu2++3SO2+6H2O==Cu2SO3CuSO32H2O��+8H++SO42- ��ֹCu2SO3CuSO32H2O �����ֽ�ͱ����� II I��III ��Һ��Ϊ��ɫ �����£���pH��ֽ(��pH��)�ⶨNaHSO3��Һ��pH����PH<7����Ka>Kb����pH>7����Ka<Kb
��������(1)�����������Ľṹ��֪����X�������Ƿ�Һ©������������������ˮ����Ũ���Ậˮ���٣�������ˮ�������ڶ�������������
��װ��CΪ��ȫƿ����������
��װ��B������ͭ������������ɲ���Cu2SO3CuSO32H2O�ķ�Ӧ��ͭԪ�ػ��ϼ��н��͵�+1�ۣ��������������Ԫ�����۵�+6�ۣ�������ӷ���ʽΪ��3Cu2++3SO2+6H2O=Cu2SO3CuSO32H2O��+8H++SO42-��
�������֪��л���ն��������ֽ⣬���ܺ����Ϊ�˷�ֹCu2SO3CuSO32H2O �����ֽ�ͱ�������
(2)��NaHSO3��Һ�м���NaClO��Һʱ����Ӧ��I��NaHSO3��NaClOǡ�÷�Ӧ��NaHSO3+NaClO=NaHSO4+NaCl������NaClO���㣺2NaHSO3+NaClO=Na2SO4+SO2��+H2O+NaCl������NaClO������NaHSO3+NaClO=Na2SO4+NaCl+HClO��
��� | ʵ����� | Ԥ�������� |
�� | �������Թ�A�еμ���ˮ������� | ����Һ��ɫ����ϲ���������������������Һ����ɫ����ϲ��������������� |
�� | ��ȡ���������Һ���Թ�B�У��μӼ��ε���KI��Һ������� | ��Һ��Ϊ��ɫ������������ |
(3)���������ԣ�ˮ���Լ��ԣ���ⶨpH���ɣ������ʵ��Ϊ�����£���pH��ֽ����pH�ƣ��ⶨNaHS03��Һ��pH����pH��7����Ka��Kb����pH��7����Ka��Kb��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ͭ���仯������й㷺��Ӧ�á��ش��������⣺
(1)CuSO4��Cu(NO3)2�������ӵĻ�̬��������Ų�ʽΪ__________��S��O��N����Ԫ�صĵ�һ�������ɴ�С��˳��Ϊ___________________��
(2)Cu(NO3)2��Һ��ͨ������NH3�����������[Cu(NH3)4](NO3)2������NH3����ԭ�ӵ��ӻ��������Ϊ________��[Cu(NH3)4](NO3)2�д��ڵĻ�ѧ�����˼��Թ��ۼ��⣬����___________��
(3)CuSO4��Һ�м������KCN������������[Cu(CN)4]2-��1mol CN�к��еĦм���ĿΪ_____________����CN-��Ϊ�ȵ������������_________(д��һ�ּ���)��
(4)CuSO4���۵�Ϊ560�棬Cu(NO3)2���۵�Ϊ115�棬CuSO4�۵���ߵ�ԭ�������_______________________________��

(5)��֪Cu2O�����ṹ��ͼ��ʾ���þ���ԭ���������AΪ(0��0��0)��BΪ(1��0��0)��cΪ![]() ����Dԭ�ӵ��������Ϊ__________��������_________ԭ��(��Ԫ�ط���)��
����Dԭ�ӵ��������Ϊ__________��������_________ԭ��(��Ԫ�ط���)��
(6)����ͭ�������������ܶѻ���ʽ��������ͭԭ�ӵ���λ����_______���þ�����Cuԭ�ӵĿռ���������__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���״���CH3OH������Ҫ���ܼ������ȼ�ϣ���ҵ����CO��H2�Ļ������Ϊԭ��һ���������Ʊ��״�
(1)�״����Ҵ���Ϊ_______________����ȫȼ��ʱ���״���ͬ���ʵ��������ͣ���ƽ�����ΪC8H18)����O2��֮��Ϊ________________��
(2)��ҵ�ϳ��á�ˮú���������CO��H2�䷴Ӧԭ�����£�
C(s)+H2O(g)![]() CO(g)+H2(g) CO(g)+H2O(g)
CO(g)+H2(g) CO(g)+H2O(g)![]() CO2(g)+H2(g)
CO2(g)+H2(g)
ijˮú����Ʒ�к�0.2LCO��0.5LCO2�������Ʒ�к�H2_________L��
(3)��ҵ�ϻ�����ͨ������;�����H2�����н���Ч����õ���_______________��
a.���·ֽ�ˮ��ȡH2��2H2O![]() 2H2��+O2��
2H2��+O2��
b.���ˮ��ȡ H2��2H2O![]() 2H2��+O2��
2H2��+O2��
c.������ˮ��Ӧ��ȡH2��CH4+H2O![]() 3H2+CO
3H2+CO
d.�ڹ���������£�����̫���ֽܷ�ˮ��ȡH2��2H2O![]() 2H2��+O2��
2H2��+O2��
(4)��2L���ܱ������г���lmoCO��2molH2�����������·�����Ӧ��
CO(g)+2H2(g)![]() CH3OH(g)
CH3OH(g)
���CO��CH3OH(g)Ũ�ȱ仯��ͼ��ʾ��

�ٴӷ�Ӧ��ʼ��ƽ�⣬������ƽ����Ӧ����v(H2)=_____mol��L-1��min-1��
���ܹ��жϸ÷�Ӧ�ﵽƽ�����_________��ѡ��)��
a.CO��H2��CH3OH�������ʵ�Ũ�����
b.�ܱ������л��������ܶȲ��ٸı�
c.CH3OH�ֽ�����ʺ�CH3OH���ɵ��������
d.��ͬʱ��������lmolCO��ͬʱ����1molCH3OH
(5)Ϊʹ�ϳɼ״�ԭ�ϵ�ԭ�������ʴ�100%��ʵ���������Ʊ�ˮú��ʱ��ʹ��CH4��������Ͷ��ʱ��n(C)��n(H2O)��n(CH4)=____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���±���Ԫ�����ڱ���һ����,��Ա��еĢ�����Ԫ�أ���д���пհף�
�� ���� | IA | IIA | ��A | IVA | VA | VIA | ��A |
1 | �� | ||||||
2 | �� | �� | �� | ||||
3 | �� | �� | �� | �� | �� | �� |
(1)����Ԫ���У��γɻ���������������_____________(��Ԫ�ط���)��
(2)Ԫ�آ١��ܺ͢��γɵĻ�����ĵ���ʽ��_______���û������д��ڵĻ�ѧ��������_______��
(3)�ڡ��ۡ�������Ԫ��ԭ�Ӱ뾶�ɴ�С��˳����___________(��Ԫ�ط��ű�ʾ)��
(4)�ݡ��ޡ�������Ԫ�����{���������Ӧˮ����ļ�����ǿ������˳����______________��(�ö�Ӧ���ʵĻ�ѧʽ��ʾ��
(5)Ԫ�آߺ͢�����������Ӧ��ˮ�������Ӧ�����ӷ���ʽΪ___________________��
(6)�ܱȽ�Ԫ�آ�͢�ǽ�����ǿ����ʵ����ʵ��_________(����ĸ���)��
a.����⻯������ԱȢ���⻯���������
b.��ĵ���R2��H2���ϱȢ�ĵ���Q��H2�������ף���HR���ȶ��Ա�H2Qǿ
c.�ڢ���⻯��H2Q��ˮ��Һ��ͨ������ĵ���R2������û�������Q
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����仯�����ڲ������졢�л��ϳɵȷ�����;�dz��㷺���ش��������⣺
(1)VB2��һ�ֵ����մɲ��ϣ���̬��ԭ�ӵļ۵����Ų�ͼΪ_______��
(2)B��C��N����Ԫ�ص�һ��������С�����˳��Ϊ________��
(3)���±�����ڹ�ҵ������Ҫ���ã��������±����ķе����±���ʾ��
BF3 | BCl3 | BBr3 | BI3 | |
�е�/K | 172 | 285 | 364 | 483 |
������±����е��������ߵ�ԭ����__________________��
����BF3���ӽṹ���ͷ�ӦBF3(g)+NH4F(s)==NH4[BF4] (s)�ܹ�������ԭ��____________��
�Ʊ�������ķ������£�
![]()
BCl3��LiBH4����ԭ�ӵ��ӻ������������Ϊ_________����B3N3H6��Ϊ�ȵ�����ķ��ӵĽṹ��ʽΪ________________��
(4)������������۵�Ϊ3000�棬�侧���ṹ��ͼ��ʾ����������a=361.5pm��
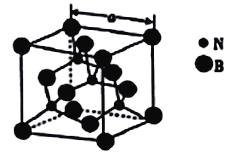
������������ľ�������Ϊ_______________��
�ڽ��ڵ�������ԭ�Ӽ�ľ���Ϊ_______(�г�����ʽ����) pm��
��������������ܶ�Ϊ_____(�г�����ʽ����)g�M-3��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ����Zn��Cu�γɵ�ԭ��أ�ijʵ����ȤС������ʵ����ڶ��鿨�ϵļ�¼��ͼ��ʾ����Ƭ�ϵ�������������


A. �٢ڢ� B. �ڢ� C. �ܢݢ� D. �ۢܢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ڷ�Ӧ2HI(g) ![]() H2(g) +I2(g),���������ܹ�˵������ƽ��״̬����
H2(g) +I2(g),���������ܹ�˵������ƽ��״̬����
A. ����������ɫ���ٱ仯
B. �¶Ⱥ����һ��ʱ��������ѹǿ���ٱ仯
C. lmolH-H�����ɵ�ͬʱ��2molH-I������
D. �����ʵ����ʵ���Ũ��֮��Ϊ2��1��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʵ��������KMnO4��Һ�ܴﵽԤ��Ŀ�ĵ���
A. ������������ B. ������ϩ�еĶ�������
C. ���𱽺ͼױ� D. ����CH2=C��CH3��CHO�к�̼̼˫��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ca��H2PO2��2��������ƣ�һԪ��ǿ��H3PO2�ĸ�������һ�ְ�ɫ�ᾧ��ĩ������ˮ������ʱ���ܽ��16.7g/100gˮ������ˮ��Һ���������ԡ�������ҽҩ�����������Լ��Ʊ��������Ƶȡ��ɰ�����P4����ʯ�����Ʊ�Ca��H2PO2��2��ʵ�鲽�����£�

����1.��������ƿ�м������ʯ���飬��ͨ��N2��Ȼ����Լ98��C�³�ֽ���1h��ͬʱ�ռ�������PH3��
����2.����ӦҺ���á����ˡ�
����3.����Һ��ͨ������CO2���ٹ��ˡ�
����4.�ô�������Һ���ڲ���3��Һ��pH��Ũ������ȴ�ᾧ������ô�����ơ�
��1������1��ͨ��N2��Ŀ���� �������Ŀ���� ��
��2������1ʯ������ P4��������������Ӧ�Ļ�ѧ����ʽΪ ������2�������������ɷ�Ϊ ��
��3������3��Ŀ���� ��
��4���벹�������ɲ�Ʒ��һ���Ʊ�NaH2PO2��H2O��ʵ�鷽����ȡ��Ʒ������Ƽ����ձ��У���������ˮ�ܽ⣬ ������õ�NaH2PO2��H2O������֪���ڳ�ѹ�£�������������������Һ�ᷢ����ը��100��ʱNaH2PO2��H2O���ܽ��Ϊ667g/100gˮ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com