
����Ŀ�����Ȼ�����S2Cl2���ǹ㷺������ҵ����������ӽṹ��ͼ��ʾ�������£�S2Cl2��һ�ֳȻ�ɫҺ�壬��ˮ��ˮ�⣬��������ʹƷ����ɫ�����塣����˵������������
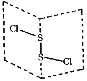
A.S2Cl2�ĽṹʽΪCl��S��S��Cl
B.S2Cl2Ϊ���м��Լ��ͷǼ��Լ��ļ��Է���
C.S2Br2��S2Cl2�ṹ���ƣ��۷е�S2Cl2��S2Br2
D.S2Cl2��H2O��Ӧ�Ļ�ѧ����ʽ����Ϊ��2S2Cl2+2H2O=SO2��+3S��+4HCl
���𰸡�C
��������
A.��ͼ֪S2Cl2�ĽṹʽΪCl��S��S��Cl��A��ȷ��
B. Cl��SΪ���Լ���S��SΪ�Ǽ��Լ����ǽ�����Cl��S���������ȴ����ָ���ɣ������������ɣ�����������IJ��ص���S2Cl2Ϊ���Է��ӣ�B��ȷ��
C. ���ǹ��ɵľ����Ƿ��Ӿ��壬�۷е�ĸߵ�ȡ���ڷ��Ӽ����������S2Br2��S2Cl2�ṹ���ƣ���Է�������Խ���Ӽ��������Խ���۷е�Խ�ߣ��۷е�S2Cl2��S2Br2��C����
D. �������Ϣ��֪��S2Cl2��H2O����ˮ�⣬���ɶ������������Ԫ�ػ��ϼ۱�۹��ɣ���Ӧ�Ļ�ѧ����ʽ����Ϊ��2S2Cl2+2H2O=SO2��+3S��+4HCl��D��ȷ��
��ѡC��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��101 kPaʱ��1molH2��ȫȼ������Һ̬ˮ���ų�285.8 kJ��������1molCH4��ȫȼ������Һ̬ˮ��CO2���ų�890.3 kJ�������������ܱ�ʾȼ���ȵ��Ȼ�ѧ����ʽ��
A.CH4(g)+2O2(g)![]() CO2(g)+2H2O(l) ��H=890.3 kJ
CO2(g)+2H2O(l) ��H=890.3 kJ
B.CH4(g)+2O2(g)![]() CO2(g)+2H2O(l) ��H=+890.3 kJ��mol1
CO2(g)+2H2O(l) ��H=+890.3 kJ��mol1
C.CH4(g)+2O2(g)![]() CO2(g)+2H2O(g) ��H=890.3 kJ��mol1
CO2(g)+2H2O(g) ��H=890.3 kJ��mol1
D.H2(g)+![]() O2(g)
O2(g)![]() H2O(l) ��H=285.8 kJ��mol1
H2O(l) ��H=285.8 kJ��mol1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ճ�����������Ľ�����ij��ͬѧ��ѧϰ����֪ʶʱ�����������⣺
����1����Ϊ�γ�Ϊ��ɫ������
����2��CuO�ڸ����¿ɷֽ�Ϊ Cu2O��O2��Fe2O3�ڸ����¿ɷֽ�ΪFeO��O2��
(1)��������1��ͬѧ���������ң������ֽ��ͣ�
A����Ϊ�����������к�ɫ��������������Խк�ɫ����
B����Ϊ���ķ�ĩΪ��ɫ������������Ҳ��Ϊ��ɫ�����Խк�ɫ����
������Ϊ��ȷ��˵����__________��
������һ��ɫ��ĩ����μ��������ۣ�����Fe3O4��______________________________________��
������һ��ɫ��ĩ��Ϊ���������������Ļ������֤��������Fe3O4(ֻҪ�����ʵ�鷽��)��____________________________________��
(2)��������2��ͬѧ����ʵ��̽�����������������ַ�����
A���������������������գ�������ǰ����ɫ�Ƿ�仯��
B���������������������գ�������ǰ�������Ƿ�仯��
��ʵ����Ӧ��Fe2O3����__________(����������)�����ա�
������A�У�����������պ���ɫ��__________��Ϊ__________��˵��Fe2O3ȷʵ�����˱仯����˵�����ɵ�һ��ΪFeO��__________��������________________________��
������B�У����������Ԥ�ڵķ�Ӧ�������������ǰ��������ӦΪ________�����ǣ�ʵ������������ǰ��������Ϊ30��29����������պ���������____________________��
���Ƚ����ַ���������Ϊ�Ϻõķ�����__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ʵ������ȡCl2����Cl2Ϊԭ�Ͻ����ض���Ӧ��ʵ�飺

��1��AΪ��������װ�ã�д����Ӧ�Ļ�ѧ����ʽ_____________________.
��2��ʵ�鿪ʼ�ȵ�ȼA���ľƾ��ƣ�������K����Cl2��������װ�ã��ٵ�ȼD���ľƾ���.Cl2ͨ��Cƿ�����D��Dװ����ʢ��̼�ۣ�����������ԭ��Ӧ������CO2��HCl(��)����д��Dװ���з�Ӧ�Ļ�ѧ����ʽ_______________.װ��C��������________________.
��3��E��ʯ����Һ��������_____________����ԭ����________________.
��4������E����Һ��Ϊ����ʯ��ˮ����Ӧ���̵�������___________.
A���а�ɫ�������� B���ް�ɫ��������
C�������ɰ�ɫ������Ȼ�������ʧ
��5��D����Ӧ��Ϻر�����K����ȥ�ƾ��ƣ��������ȵ����ã�A����������Cl2��������ʱB�е�������________________________________________.B��������___________________________________________.
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��Ӧ��H2(g)��![]() O2(g)=H2O(g) ��H1
O2(g)=H2O(g) ��H1
![]() N2(g)��O2(g)=NO2(g) ��H2
N2(g)��O2(g)=NO2(g) ��H2
![]() N2(g)��3/2H2(g)=NH3(g) ��H3
N2(g)��3/2H2(g)=NH3(g) ��H3
��Ӧ2NH3(g)��![]() O2(g)=2NO2(g)��3H2O(g)����HΪ�� ��
O2(g)=2NO2(g)��3H2O(g)����H�� ��
A. 2��H1��2��H2��2��H3B. ��H1����H2����H3
C. 3��H1��2��H2��2��H3D. 3��H1��2��H2��2��H3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵����ȷ���� �� ��
A.�������Ե��ԭ��ΪH2+2NiO(OH)=2Ni(OH)2����õ�ظ�����ӦʽΪ��H2��2e��+2OH��=2H2O
B.�ڲⶨ�к��ȵ�ʵ���У��������ʵ���һ��Ҫ���������������ʵ������������ܱ�֤���������к���ȫ
C.��֪2H2��g��+O2��g��=2H2O(g)����H=-483.6kJ/mol����������ȼ����Ϊ241.8kJ/mol
D.��֪S��s��+O2(g)=SO2(g)����H1 S(g)+O2(g)=SO2(g)����H2������H1����H2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪һ��c(H��)��1��10��3mol/L�����һ��c(OH��)��1��10��3mol/L�ļ���Һ�������Ϻ���Һ�����ԣ���ԭ�������( )
A��Ũ��ǿ���ϡ��ǿ����Һ��Ӧ
B��Ũ�������ϡ��ǿ����Һ��Ӧ
C����Ũ�ȵ�ǿ���������Һ��Ӧ
D��������һ��ǿ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��C60�����ʯ��ʯī�Ľṹģ����ͼ��ʾ(ʯī����ʾ�����е�һ��ṹ)
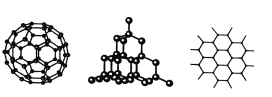
(1)C60�����ʯ��ʯī���ߵĹ�ϵ��Ϊ_________
A.ͬ���칹�� B.ͬ�������� C.ͬϵ�� D.ͬλ��
(2)��̬ʱ��C60����__________����(��������������ԭ��������������)��
(3)�辧��Ľṹ�����ʯ���ƣ�1 mol�辧���к��й�ĵ�������ĿԼ��________NA������������Ľṹ�൱���ڹ辧��ṹ��ÿ���赥��֮�����1����ԭ�ӡ���������Ŀռ���״�ṹ�У�����ԭ���γɵ���С������ԭ�ӵ���Ŀ��__________��
(4)ʯī��״�ṹ�У�ƽ��ÿ����������ռ�е�̼ԭ������__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪2FeSO4![]() Fe2O3��SO2����SO3����ijͬѧ���������ͼװ�÷ֱ��������е����塣�����йر���������ǣ� ��
Fe2O3��SO2����SO3����ijͬѧ���������ͼװ�÷ֱ��������е����塣�����йر���������ǣ� ��
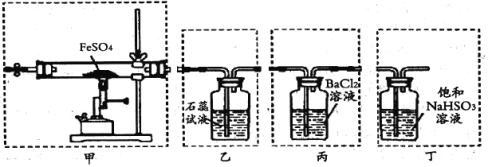
A.��װ�ü��·ֽ�FeSO4����ȼ�ƾ����ǰӦ����װ����ͨһ��ʱ��N2
B.��װ���ҿɼ���ֽ������SO2��������ʯ����Һ�ȱ�����ɫ
C.���ռ�������������������˳����װ�ñ�����ֽ������SO3
D.��װ�ö��е��Լ���ΪNaOH��Һ�ܸ��õı�����Ⱦ����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com