
����Ŀ��̼�������仯������ͬѧ�Ǿ����ܽӴ�������Ҫ���ʣ��ǿ�ѧ�о�����Ҫ����
(1)ʵ������ȡ��Ȳ�Ļ�ѧ����ʽΪ___________________________��
(2)H2NCOONH4�ǹ�ҵ�ϳ����ص��м����÷�Ӧ�������仯��ͼA��ʾ����CO2�Ͱ����ϳ����ص��Ȼ�ѧ����ʽΪ___________________________��

(3)��������CO2��CH4����������ЧӦ��Ϊ��ѧ�о������ȵ㡣һ���Զ������ѱ��渲��Cu2A12O4Ϊ���������Խ�CO2��CH4ֱ��ת�������ᣨ��H<0)���ڲ�ͬ�¶��´����Ĵ�Ч����������������ʷֱ�����ͼB��ʾ��250��300��ʱ���¶����߶�������������ʽ��͵�ԭ����________________��250���400��ʱ������������ʼ�����ȣ�ʵ��������Ӧѡ����¶�Ϊ_________�档
(4)T��ʱ���������ʵ�����NO��CO�������Ϊ2L�� �ܱ������з�����Ӧ2NO+2CO![]() 2CO2+N2�������¶Ⱥ�������䣬��Ӧ������NO�����ʵ�����ʱ��ı仯��ͼC��ʾ��
2CO2+N2�������¶Ⱥ�������䣬��Ӧ������NO�����ʵ�����ʱ��ı仯��ͼC��ʾ��

��ƽ��ʱ�������¶Ȳ��䣬���������г���CO��N2��0.8mol��ƽ�⽫______(����������ҡ��������ƶ���
��ͼ��a��b�ֱ��ʾ��һ���¶��£�ʹ����ͬ��������ͬ������Ĵ���ʱ���ﵽƽ�������n(NO)�ı仯���ߣ����б�ʾ����������ϴ��������______(�a����b��)��
��15minʱ,���ı���練Ӧ����������n(NO)������ͼ��ʾ�ı仯����ı������������_____________(�δ�һ������)��
(5)��������Һ�к��д����İ������ʣ���NH3��ʾ�����Ȼ�����õ��ԭ������Һ�еİ���������ȫ������ȥ���ù��̷�Ϊ��������һ�����������������ڶ���������������������������ΪN2��
�ٵڶ�����Ӧ�Ļ�ѧ����ʽΪ____________________��
������������Һ�а������ʵ���������Ϊ0. 034% ���������õ�ⷨ����It����ˮ��
��·��ת�Ƶĵ�����Ϊ__________��
���𰸡� CaC2 + 2H2O = Ca(OH)2 + C2H2�� 2NH3(g) + CO2(g)==CO(NH2)2(s)+ H2O(1)��H=-134 kJ/mol 250�棬�����Ĵ�Ч����ã�֮������Ĵ�Ч�ʼ��罵�� 250�� ���� b ����CO�����ʵ���Ũ�ȡ�����ѹǿ������������Ũ�� 3Cl2+2NH3==N2+6HCl 3.612��1025 (��60NA)
��������(1)ʵ�����õ�ʯ��ˮ��Ӧ��ȡ��Ȳ����ѧ����ʽΪ (1). CaC2 + 2H2O = Ca(OH)2 + C2H2��
��2����Ӧ�������ߣ������������ͣ���ͼ��֪�ų�������Ϊ��272kJ/mol -138kJ/mol =134kJ/mol ����CO2�Ͱ����ϳ����ص��Ȼ�ѧ����ʽΪ2NH3(g) + CO2(g)==CO(NH2)2(s)+ H2O(1)��H=-134 kJ/mol (3)250��300��ʱ���¶����߶�������������ʽ��͵�ԭ���ǣ�250�棬�����Ĵ�Ч����ã�֮������Ĵ�Ч�ʼ��罵�ͣ�250���400��ʱ������������ʼ�����ȣ�ʵ��������Ӧѡ����¶�Ϊ250�棬250��ʱ����������ߡ�(4)��ʼNOΪ0.4molƽ��ʱΪ0.2mol

ƽ��ʱŨ��Ϊ0.1mol/L��0.1mol/L��0.1mol/L��0.05mol/L

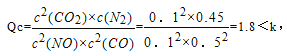 �ʷ�Ӧ���ҽ��С�
�ʷ�Ӧ���ҽ��С�
�ڴ����������Ӧ���ʿ죬��ƽ��ʱ��̣���ͼ��֪��b���ߴ��������µķ�Ӧ���ʿ죬b�Ĵ������������ͼ���֪��NO��Ũ�ȼ�С��ƽ�������ƶ������Ըı�����Ϊ����CO�����ʵ���Ũ�ȡ�����ѹǿ������������Ũ�� ��
(5)�ڶ���������������������������ΪN2������ʽΪ��3Cl2+2NH3==N2+6HCl��n(NH3)=106g��0. 034%/17g��mol-1==20mol,N��-3�۱��0�ۣ�ת�Ƶ�����3.612��1025 (��60NA)


 ��һ����ͬ���ɽ�����ϵ�д�
��һ����ͬ���ɽ�����ϵ�д� ������Ӧ���ϵ�д�
������Ӧ���ϵ�д� ��ʦ�㾦�ִʾ��ƪϵ�д�
��ʦ�㾦�ִʾ��ƪϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ڱ�״���£���������������ɵĻ�������干560 mL��������ͨ��H2����һ�������£�ʹ��ǡ����ȫȼ�գ���ˮ�������õ�������Ƶ�250 mL��Һ������ȡ��25 mL��Һ��20 mL 0.125 mol��L��1����������Һ��Ӧǡ���кͣ����������巴Ӧ��H2�����Ϊ�� ��
A. 840 mL B. 720 mL C. 500 mL D. 280 mL
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ�ҷ�����ij��Ԫ�ص�һ����ԭ�ӣ���������a g��12Cԭ�ӵ�������b g��NA�ǰ����ӵ�������ֵ������˵������ȷ����
A������֪��Ϣ�ɵ�NA��![]()
B��w g��ԭ�ӵ����ʵ���һ����![]() mol
mol
C��w g��ԭ���к���![]() ��ԭ��
��ԭ��
D����Ԫ�ص�Ħ��������aNA g��mol��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Na2S2O5��������֯ҵ�������ȼ����ữʱ�ɷų�SO2���塣���Ʊ���������
���£�
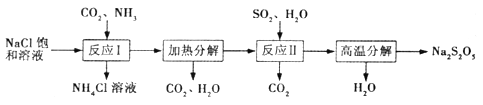
(1)Na2S2O5������Ϊ_______________(ѡ��������ơ����������ơ�)��
(2)����ӦI ����Ӧ��ͨ���������______����Ӧ�������Ĺ��������____________��
(3)����ӦII���Ļ�ѧ����ʽΪ_________________________��
(4)����Ӧ�����������ȿ��Ʋ����������õ�Na2S2O5��Ʒ�������������ʡ�
������Ʒ�к�������Na2SO3�������ԭ�������______________(�δ�һ������);
���������Ʒ�к�������Na2SO3�������Լ���ʹ��˳������Ϊ_________(����)��
a.ϡ���� b.�Μ[ʯ��ˮ c.Ʒ����Һ d.����KMnO4��Һ
(5)Na2S2O3Ҳ��������������ȼ���
��Na2S2O5��Һ��Cl2��Ӧ�����ӷ���ʽΪ____________________��
��Na2S2O5��Na2S2O3���ȵ�Чʱ�����Ķ��ߵ�����֮��Ϊ____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������14.4 g CO��CO2�Ļ�����壬�ڱ�״������ռ�����ԼΪ8.96 L���ش��������⣺
��1���û�������ƽ��Ħ��������_______________________��
��2�����������̼ԭ�ӵĸ�����____________________��(��NA��ʾ�����ӵ�������ֵ)
��3���������������ͨ����ͼװ�ã�����ռ��������С�
���������ռ������������Է���������________________��
�ڱ�״�����������ռ�������������Ϊ____________��
���������ռ���������ĵ�������Ϊ_______________________��(��NA��ʾ�����ӵ�������ֵ)

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����100 mL 1.5 mol��L1ϡ�����м������Mg�ۣ���ַ�Ӧ����ȥ����Mg�ۣ���Һ��t ���º�������������Һ����Ϊ72.0 gʱ��ʼ����MgSO4��xH2O���壬����������12.3 gʱ��ʣ����Һ48.0 g��ͨ������ش��������⡣
��1���������ɱ�״���µ��������(��д���������)��
��2����ʼ����MgSO4��xH2O����ʱ��Һ����������Ϊ ��
��3��MgSO4��xH2O�е�x= ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪����ʵ����ʵ��
��Cr2O3�����������KOH��Һ�õ�KCrO2��Һ��������������õ�Cr2(SO4)3��Һ��
����KCrO2��Һ�еμ�H2O2��Һ�����ữ���ɵ�K2Cr2O7��Һ��
�۽�K2Cr2O7��Һ�μӵ����ۺ�KI�Ļ����Һ�У���Һ������
�����жϲ���ȷ����(����)
A. ������KCrO2��Cr�Ļ��ϼ�Ϊ��3
B. ʵ���֤��Cr2O3������������
C. ʵ���֤��H2O2�������������л�ԭ��
D. ʵ���֤�������ԣ�Cr2O![]() >I2
>I2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ƤЬ��æ���Ķ����������������磬��¶��ijЩ��ҵ����ƤЬ�������̻��ҵIJ�����Ϊ�����ӡ�ÿ���������桷�ع���һЩ������ҵ��Ƥ����ϰ��Ƴɹ�ҵ���������۸�ijЩ��ҩ��ҵ�����ձ��ҩ�ý��ҡ��ɹ�ҵ�����ƳɵĽ����������г�����ؽ��������Ӷ�����������˺���������ˮ���Ե����ʻ�������ˮ�γɽ��塣
��1����֪Fe(CrO2)2�и�Ԫ����+3�ۣ���������Ԫ����___________�ۡ�![]() ��һ��������ӣ���Fe(CrO2)2����__________(��ᡱ����������Ρ��������)��
��һ��������ӣ���Fe(CrO2)2����__________(��ᡱ����������Ρ��������)��
��2��������ˮ��Һ��K2SO4��Һ��ͬ�߱���������_____________(�����)��
a�������ȶ����ܷ���ó���
b�����߾��ж����ЧӦ
c����ɢ�����ӿ�ͨ����ֽ
��3����֪����ķ�ɢ�ʲ�������Ĥ����ˮ���ӵ�С���ӻ�����������Ĥ���ᴿ������װ���������е�____________(�����)��

��4������10 mL������ˮ��Һ��5 mL Na2SO4��Һ���װ���Ĥ�ڣ����˰�Ĥ������ʢ����ˮ���ձ��У����ʵ��֤��![]() �ܹ�����Ĥ��____________________________________��
�ܹ�����Ĥ��____________________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com